Bénédicte Colnet brilliantly defended her PhD on generalization of causal effects across populations on June 29th 2023.
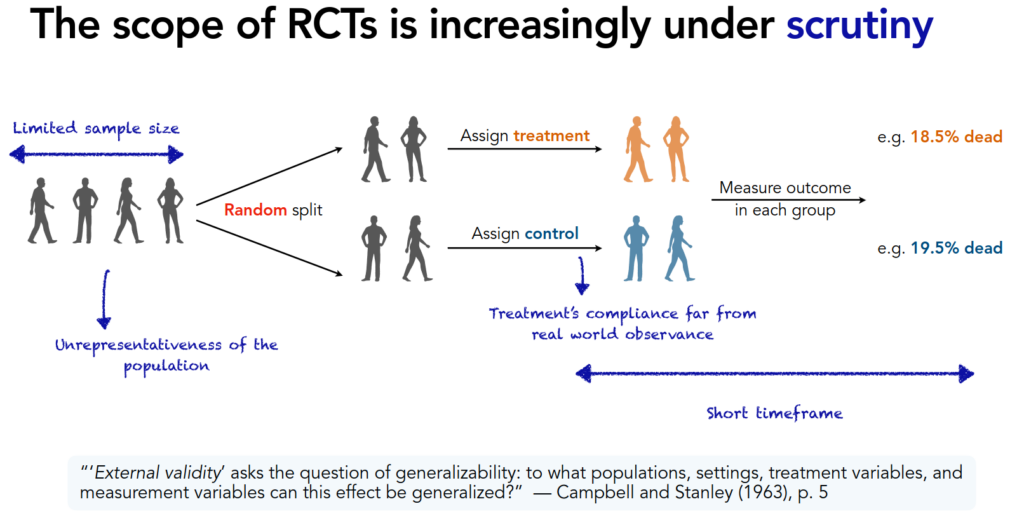
The question she tackled is the following: to empirically evaluate a causal effect, randomized controlled trials (RCTs) give excellent control of confounding effects (internal validity), but they often are performed on a population different from the target population of interest (for instance because vulnerable people are not included).
Bénédicte studied how to combine trial data with data on the target population to estimate the effect on the target population. Her work led to the following publications:
- Causal inference methods for combining randomized trials and observational studies: a review
- Causal effect on a target population: a sensitivity analysis to handle missing covariates
- Reweighting the RCT for generalization: finite sample error and variable selection
- Risk ratio, odds ratio, risk difference… Which causal measure is easier to generalize?
Thesis abstract Modern evidence-based medicine places Randomized Controlled Trials (RCTs) at the forefront of clinical evidence. Randomization enables the estimation of the average treatment effect (ATE) by eliminating the confounding effects of spurious or unwanted associated factors.
More recently, concerns have been raised on the limited scope of RCTs: stringent eligibility criteria, unrealistic real-world compliance, short timeframe, limited sample size, etc. All these possible limitations threaten the external validity of RCT studies to other situations or populations.
The usage of complementary non-randomized data, referred to as observational or from the real world, brings promises as additional sources of evidence.
Today, there is a growing incentive to rely on these new data, which is also endorsed by health authorities such as the Food and Drug Administration (FDA) in the U.S. and the Haute Autorité de la Santé (HAS) in France.
Combining both data types -randomized and observational- is a new agenda that could make the most of both worlds.
First, this thesis proposes a review of all the existing methods combining several data types to build clinical evidence. Then, the thesis is focused on improving the external validity of RCTs.
In other words, how can we use representative sample of the target population of interest to re-weight or to generalize the trial’s findings?
Such methods are quite recent and have been proposed in the early 2010’s.
This thesis investigates theoretical properties of these methods, such as finite and large sample properties (bias and variance) of the estimation, which helps to provide practical guidelines about covariates selection and the impact of both samples’ sizes. This thesis also proposes a sensitivity analysis when covariates are either partially or totally unobserved.
Most -if not all- current statistical works concern the generalization of the effect on the scale of the absolute difference, while our clinicians collaborators pointed to us the need to encompass several causal measures (e.g. ratio, odds ratio, number needed to treat).
Therefore, this thesis also opens the door to the generalization of all causal measures of interest.
Doing so, we link generalization with a rather old concerns of causality, namely collapsibility of a measure.
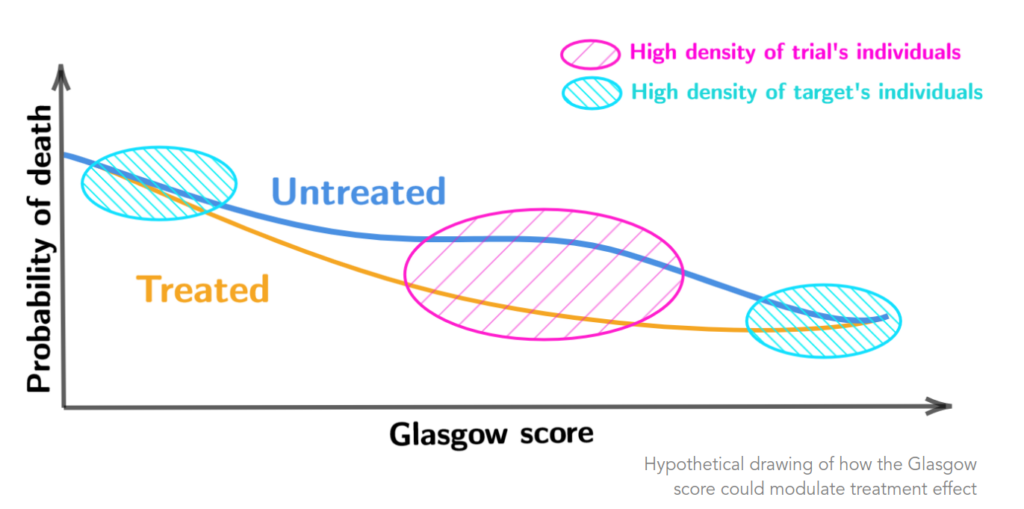
We also propose a new framing to apprehend heterogeneity of a treatment effect.
Finally, it turns out that assumptions required for generalization depends on the nature of the outcome and the causal measure of interest.
All our research questions are motivated by clinical applications, and in particular by the Traumabase consortium.

