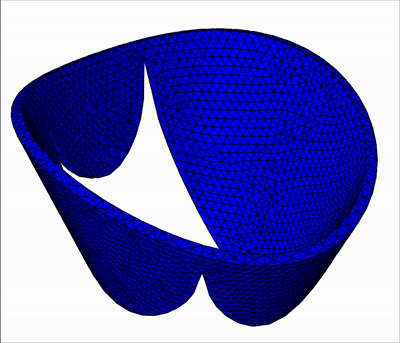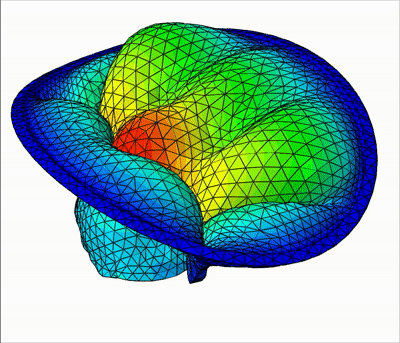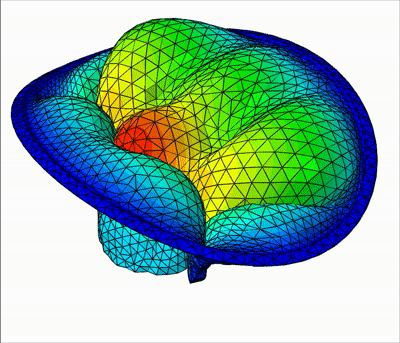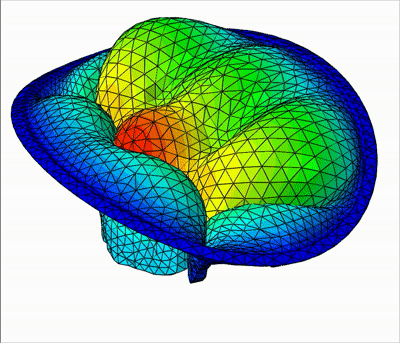
Overview of talks:
- Pierre-Frederic Villard, Nov 25th 2024,Mitral Valve Modeling: From Medical Image Analysis to Fluid Structure Interaction
- Pierre-Frederic Villard, Nov 21th 2024, Modeling using the Cosserat Model
- Hao Gao, Dec 8th 2023, Biomechanical modelling of heart function towards clinical translation: myocardium, valves, blood flow and their interactions
- Pierre-Frederic Villard, Nov 9th 2023, New contributions in mitral valve simulation
- Pierre-Frederic Villard, Nov 17th 2022, Mitral Valve Modeling: From Medical Image Analysis to Fluid Structure Interaction
- Douglas Perrin, October 10th 2022, Desktop to bedside: Bespoke application development to translate computer science to clinical tools
- Peter E. Hammer, October 10th 2022, Quantitative approaches for preoperative planning in aortic valve repair in children
- Nariman Khaledian, January 14th 2022, Capturing Contact in Mitral Valve Dynamic Closure with Fluid-Structure Interaction Simulation
- Pierre-Frederic Villard, Oct 21th 2021, Deformation Computation using RBF
- Pierre-Frederic Villard, May 18th 2021, Augmented and Virtual Reality in School Education
- Pierre-Frederic Villard, Feb 23rd 2021, 3D Lagrangian for Mitral Valve
- Pierre-Frederic Villard, Oct 27th 2020, CNN Applications on Medical and Industrial Images
- Pierre-Frederic Villard, Jun 24th 2020, Segmentation with Active Contours
- Sebastian Baudelet, Apr 29th 2020, Modelling of the Mitral Valve Closure in 2D
- Pierre-Frederic Villard, Dec 3rd 2019, An Overview of Deformable Models
- Daryna Panicheva, , Nov 19th 2019, Physically-coherent Extraction of Mitral Valve Chordae
- Douglas Perrin, September 25th 2019, Patient-Specific Mitral Valve Segmentation from 4D Ultrasound.
- Peter E. Hammer, July 1st 2019, Computational fluid dynamics to guide care of patients with congenital heart disease.
- Pierre-Frederic Villard , Nov 26th 2018, Mitral Valve modeling: from CT scan FEM model
- Daryna Panicheva , Nov 19th 2018,Automatic Segmentation of Mitral Valve Chordae
- Robert D. Howe, June 18th 2018, Fixing the Beating Heart: Ultrasound Guidance for Robotic Intracardiac Surgery.
- Peter E. Hammer, June 6th 2018,
Using engineering and computational methods to inform surgery for congenital heart disease. - Pierre-Frederic Villard , Oct 31th 2017, Automatic Reconstruction of Mitral Valve Chordae
- Thomas Waite, June 6th 2017, Cosserat Rods for Modeling Robotic Catheter Systems
- Douglas Perrin, May 30th 2017, Machine Learning and Image Processing: from the Lab to Clinical Use
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
MIT, Nov 25th 2024, 11am, building e25
Mitral Valve Modeling: From Medical Image Analysis to Fluid Structure Interaction
In this presentation, I will discuss our research on mitral valve modeling to improve surgical repair. First, I will introduce an automated pipeline for extracting valve geometry from medical images, focusing on the chordal architecture using segmentation and model-fitting techniques. Next, I will present a fluid-structure interaction model that simulates valve closure using the immersed boundary method, addressing challenges like contact handling and ensuring complete valve closure.
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
Harvard University, Nov 21th 2024, 11:00am at 110 Western Avenue, Allston
Modeling using the Cosserat Model
In this presentation, I will discuss our ongoing research on the Cosserat model within the context of thrombectomy procedures. I will outline the medical motivation for selecting the Cosserat model and its relevance to our application. Subsequently, I will detail our approaches for solving the model’s numerical equations, focusing on three techniques: the shooting method, the collocation method, and a reduction-based approach. Finally, I will present results obtained from real patient data.
Hao Gao, University of Glasgow
Inria Grand Est, December 8th 2023, 10am Room C103
Biomechanical modelling of heart function towards clinical translation: myocardium, valves, blood flow and their interactions
Heart diseases remain a leading cause of mortality worldwide, emphasizing the urgent need for effective prevention, management, and treatment strategies. Personalized biomechanical modelling of heart function plays a crucial role in understanding the complex interactions within the cardiac system, providing valuable insights into disease progression, treatment response, and potential interventions. Many existing heart models in the literature focus on electromechanical aspect without considering physiological valves or use simplified fluid models instead. Moreover, those models pose tremendous challenges when calibrated using routinely available clinical measurements. In the first part of this talk, I would like to present our research on modelling heart function using an immersed boundary methods with finite element extension, allowing for naturally integrating blood-myocardium-valve interaction by incorporating anisotropic hyperelastic constitutive laws for soft biological tissues. In a series of studies, we have demonstrated that this IB/FE framework can be used to study the versatility of this IB/FE framework in examining complex interactions among ventricles, atria, valves, and pulmonary/systemic circulations under both physiological and pathological conditions. In the second part, I will share our recent work on personalized biomechanical cardiac modelling by leveraging clinical images, and further combined with machine-learning approaches (Gaussian process, Graph Neural Network) for real-time decision-making in clinic. We aim to bridge the gap between sophisticated and computationally expensive biomechanical modelling and practical, data/physics-driven applications, paving the way for advancements in personalized cardiac care.
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
Harvard University, Nov 9th 2023, 11:00am, 4.307/4.308 at 110 Western Avenue, Allston
New contributions in mitral valve simulation
 I will present my current work on mitral valve modelling. First I will summarise the past work on segmentation and modelling of a generic valve with a fluid-structure interaction model of the valve closure under blood flow.
I will present my current work on mitral valve modelling. First I will summarise the past work on segmentation and modelling of a generic valve with a fluid-structure interaction model of the valve closure under blood flow.
I will then focus on the current work that includes working on real valves extracted from medical images and testing the impact of using an anisotropic constitutive law instead of a linear model. We used image-based model to investigate the influence of additional factors impactin closure, including leaflet smoothness and fluid volume. We used four image-based geometries for our simulations. We tested a fitted tube, representing a simplified left ventricle, it did not yield proper closure in some patients, but using a large cylinder resulted in effective valve closure. In addition, using a large cylinder provided a better coaptation area compared to the fitted tube. Finally, we investigated alternative approaches to experimentally obtaining the microstructure of the leaflet tissue. These approaches, which do not require any specific microscopic imagery, take into account the mechanical response of the tissue in the closed state, and physiological traits, such as chordae insertion points.
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
Harvard University, Nov 17th 2022, 11:00am, 4.307/4.308 at 110 Western Avenue, Allston
Mitral Valve Modeling: From Medical Image Analysis to Fluid Structure Interaction
 In this talk, I will present my current work on mitral valve modeling. The mitral valve is one of the heart valves and many pathologies damage it, potentially leading to heart failure. The aim of this work is to study tools to help surgical repair of the valve. A first study has been done on the extraction of the valve geometry from medical images. I will present a fully-automatic pipeline to extract the valve chordae architecture compatible with a computational model. First, an initial segmentation is obtained by a sub-mesh topology analysis and a RANSAC-like model-fitting approach. Then the chordal structure is optimized with respect to objective functions based on mechanical, anatomical and image-based considerations. I will then present a fluid-structure interaction model of the valve closure under blood flow. Due to the highly non-linear nature of the problem, capturing contact in fluid-structure interaction simulation is a challenging task. I will present a model based on the immersed boundary method that captures a map of contact and perfect closure of the mitral valve, without the presence of orifice holes, which often appear with existing methods.
In this talk, I will present my current work on mitral valve modeling. The mitral valve is one of the heart valves and many pathologies damage it, potentially leading to heart failure. The aim of this work is to study tools to help surgical repair of the valve. A first study has been done on the extraction of the valve geometry from medical images. I will present a fully-automatic pipeline to extract the valve chordae architecture compatible with a computational model. First, an initial segmentation is obtained by a sub-mesh topology analysis and a RANSAC-like model-fitting approach. Then the chordal structure is optimized with respect to objective functions based on mechanical, anatomical and image-based considerations. I will then present a fluid-structure interaction model of the valve closure under blood flow. Due to the highly non-linear nature of the problem, capturing contact in fluid-structure interaction simulation is a challenging task. I will present a model based on the immersed boundary method that captures a map of contact and perfect closure of the mitral valve, without the presence of orifice holes, which often appear with existing methods.
Douglas Perrin, Harvard Biorobotics Lab
Inria Grand Est, October 10th 2022, 10am Room C103
Desktop to bedside: Bespoke application development to translate computer science to clinical tools
In this talk, I will review the process of moving computer science to clinical applications, what I call Desktop-to-Bedside (a play on Bench-to-Bedside), and the challenges faced by conducting software development inside a hospital vs. a more traditional technical environment. I will sketch out a pipeline for Desktop-to-Beside and give a high-level description of several projects, how far they have made it through this pipeline, and their pain points.
Peter E. Hammer, Harvard Biorobotics Lab
Inria Grand Est, October 10th 2022, 10am Room C103
Quantitative approaches for preoperative planning in aortic valve repair in children
 Surgical repair of heart valves remains among the most challenging procedures in cardiovascular surgery.Patient-specific computer simulation has been developed as a potential tool to enable surgeons to test proposed valve repair strategies prior to surgery in order to achieve more reliable repairs and better outcomes. Many groups worldwide have been making steady progress developing these image-based computational technologies over decades. Despite this progress, these technologies have not yet matured to the point of clinical utility, leaving surgeons to continue to rely on clinical experience and subjective methods in their day-to-day surgical practice. In this talk, I will present efforts that live in the middle ground between image-based computational surgical planning and the current approach of improvising in the OR based on clinical judgement. These emerging, quantitative methods rely on preoperative or intraoperative measurements of valve features and simple, conceptual models of valve relationships and function.
Surgical repair of heart valves remains among the most challenging procedures in cardiovascular surgery.Patient-specific computer simulation has been developed as a potential tool to enable surgeons to test proposed valve repair strategies prior to surgery in order to achieve more reliable repairs and better outcomes. Many groups worldwide have been making steady progress developing these image-based computational technologies over decades. Despite this progress, these technologies have not yet matured to the point of clinical utility, leaving surgeons to continue to rely on clinical experience and subjective methods in their day-to-day surgical practice. In this talk, I will present efforts that live in the middle ground between image-based computational surgical planning and the current approach of improvising in the OR based on clinical judgement. These emerging, quantitative methods rely on preoperative or intraoperative measurements of valve features and simple, conceptual models of valve relationships and function.
Nariman Khaledian, Université of Lorraine – Loria – Inria
Jan 14th 2022, 6pm, videoconference from Nancy
Capturing Contact in Mitral Valve Dynamic Closure with Fluid-Structure Interaction Simulation
 Realistic FSI simulation of the mitral valve opens the way toward planning for surgical repair. In the literature, blood leakage is identified by measuring the flow rate but detailed information about closure efficiency is missing. We present in this paper an FSI model that improves the detection of blood leakage by building a map of contact. Our model is based on the immersed boundary method that captures a map of contact and perfect closure of the mitral valve, without the presence of orifice holes, which often appear with existing methods. We also identified important factors influencing convergence issues. The method is demonstrated in three typical clinical situations: mitral valve with leakage, bulging, and healthy. In addition to the classical ways of evaluating MV closure, such as stress distribution and flow rate, the contact map provides easy detection of leakage with identification of the sources of leakage and a quality assessment of the closure. Our method significantly improves the quality of the simulation and allows the identification of regurgitation as well as a spatial evaluation of the quality of valve closure. Comparably fast simulation, ability to simulate large deformation, and capturing detailed contact are the main aspects of the study.
Realistic FSI simulation of the mitral valve opens the way toward planning for surgical repair. In the literature, blood leakage is identified by measuring the flow rate but detailed information about closure efficiency is missing. We present in this paper an FSI model that improves the detection of blood leakage by building a map of contact. Our model is based on the immersed boundary method that captures a map of contact and perfect closure of the mitral valve, without the presence of orifice holes, which often appear with existing methods. We also identified important factors influencing convergence issues. The method is demonstrated in three typical clinical situations: mitral valve with leakage, bulging, and healthy. In addition to the classical ways of evaluating MV closure, such as stress distribution and flow rate, the contact map provides easy detection of leakage with identification of the sources of leakage and a quality assessment of the closure. Our method significantly improves the quality of the simulation and allows the identification of regurgitation as well as a spatial evaluation of the quality of valve closure. Comparably fast simulation, ability to simulate large deformation, and capturing detailed contact are the main aspects of the study.
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
Oct 21th 2021, 6pm, videoconference from Nancy
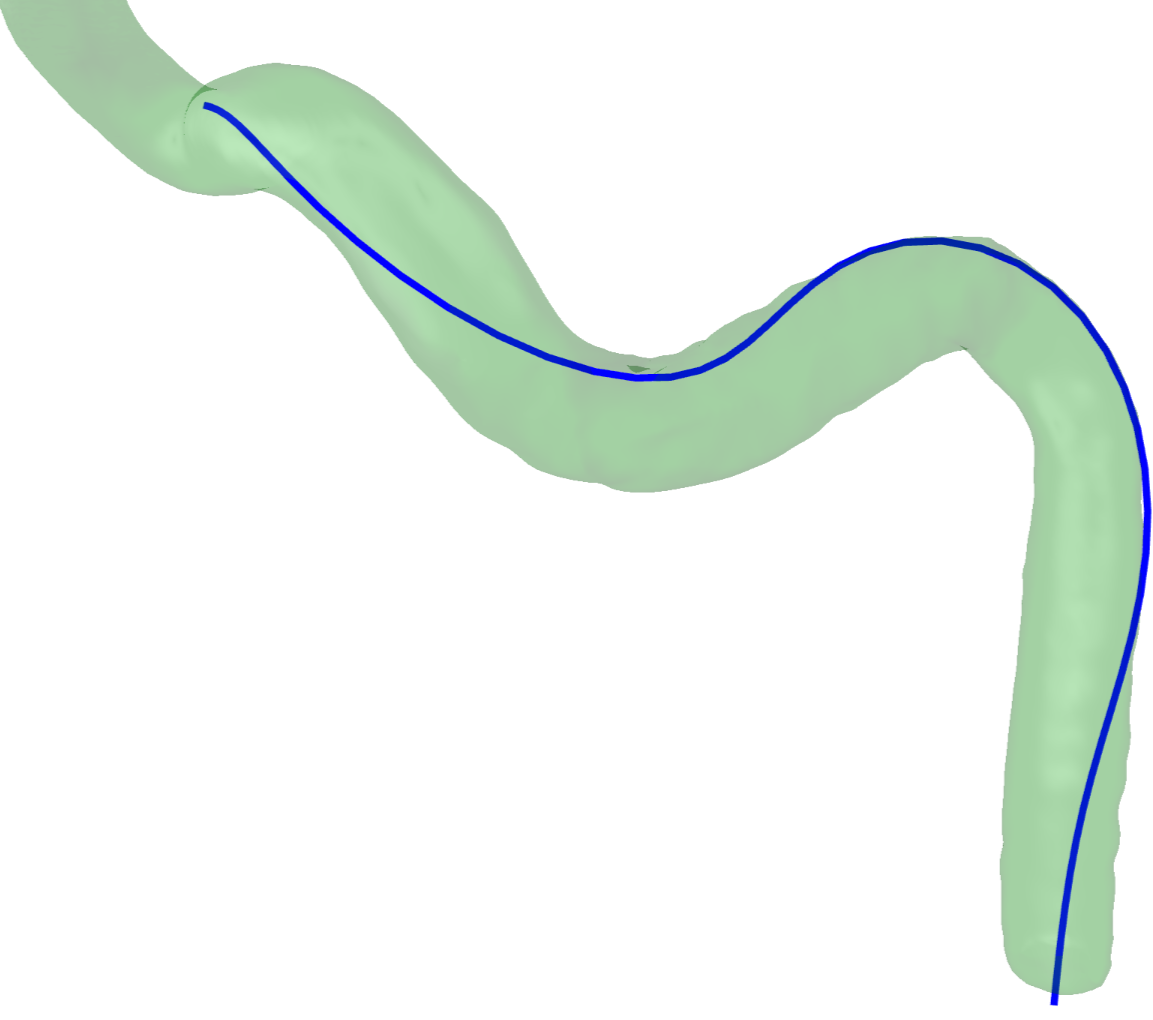
Deformation Computation using RBF
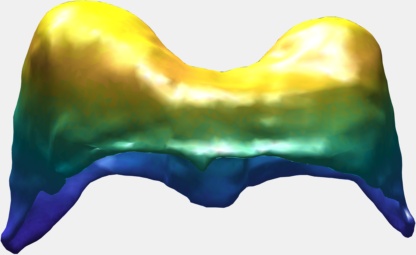 Various methods exist to compute deformation. Accurate methods such as FEM-based methods are accurate but often require significant computing time and other methods such as the mass-spring methods are fast but not as physically realist. I will present a formulation based on Radial Basis Functions where the problem is modeled with small deformation, linear elasticity and within the application of the diaphragm deformations. Results will show that our method is faster than an equivalent FEM-based simulation.
Various methods exist to compute deformation. Accurate methods such as FEM-based methods are accurate but often require significant computing time and other methods such as the mass-spring methods are fast but not as physically realist. I will present a formulation based on Radial Basis Functions where the problem is modeled with small deformation, linear elasticity and within the application of the diaphragm deformations. Results will show that our method is faster than an equivalent FEM-based simulation.
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
May 18th 2021, 6pm, videoconference from Nancy
Augmented and Virtual Reality in School Education
 I will introduce in this talk the work I have been doing with bachelor and master students in order to produce innovative pedagogical tools for secondary schools. The work environment includes Unity for the software and hardwares such as VR and cardboard headsets, tablet, smartphones and webcams. Various computer graphics considerations will be overviewed in the different applications produced during the student projects: texturing, lighting, animation and 3D modeling. The method used to produce augmented reality will also be detailed. Finally, experiments that has been done in middled school will be presented and analysed.
I will introduce in this talk the work I have been doing with bachelor and master students in order to produce innovative pedagogical tools for secondary schools. The work environment includes Unity for the software and hardwares such as VR and cardboard headsets, tablet, smartphones and webcams. Various computer graphics considerations will be overviewed in the different applications produced during the student projects: texturing, lighting, animation and 3D modeling. The method used to produce augmented reality will also be detailed. Finally, experiments that has been done in middled school will be presented and analysed.
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
Feb 23rd 2021, 6pm, videoconference from Nancy
3D Lagrangian for Mitral Valve
 Lagrangian formulation in mechanics allow to compute geometrical configurations at equilibrium. In this work, a 3D framework has been developed taking into account Internal forces of the mitral valve leaflets, the blood pressure, the chordae tension forces and the contact forces. Equilibrium is found when the Lagrangian in minimum. A technique based on gradient descent has been developed to find the best deformed mesh. It is based on analytic expressions of the energies and the code has been optimized to be real time. Results include different applications: balloon inflation, parachute with air pressure and of course, mitral valve.
Lagrangian formulation in mechanics allow to compute geometrical configurations at equilibrium. In this work, a 3D framework has been developed taking into account Internal forces of the mitral valve leaflets, the blood pressure, the chordae tension forces and the contact forces. Equilibrium is found when the Lagrangian in minimum. A technique based on gradient descent has been developed to find the best deformed mesh. It is based on analytic expressions of the energies and the code has been optimized to be real time. Results include different applications: balloon inflation, parachute with air pressure and of course, mitral valve.
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
Oct 27th 2020, 3pm, videoconference from Nancy
CNN Applications on Medical and Industrial Images
 Neural network are used for image recognition. One input image goes through the neural network, which gives a prediction of it. At the opposite, the segmentation of an image is a prediction of each pixel.
Neural network are used for image recognition. One input image goes through the neural network, which gives a prediction of it. At the opposite, the segmentation of an image is a prediction of each pixel.
The purpose of image segmentation is to highlight one or many regions of the image.
I will present two applications: 1) default detection in an industrial context of metal fabrics used for filtering and 2) diaphragm segmentation on medical data in order to extract a mesh to build a surgical simulator. The robustness of the neural networks has been tested, as well as the impact of the normalization variation.
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
Jun 24th 2020, 6pm, videoconference from Nancy
Segmentation with Active Contours
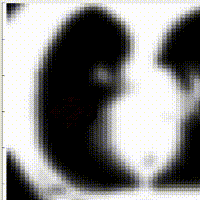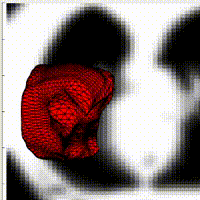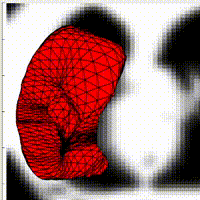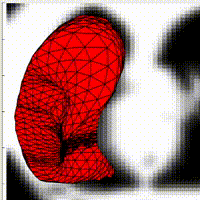 Active contours have shown their ability to introduce regularity on binary image segmentation. In contrast with level-set approaches, they are able to maintain the initial topology of the area of interest. For this reason, it has been used in recent medical research for diaphragm segmentation. Most of the online codes for 3D segmentation, as well as built-in Matlab toolboxes, are based on level-set methods. Moreover, in the literature, the implementation details of active contours methods with meshes in three dimensions are tight, making tedious any reproduction of these techniques. In this talk, I will present some details on the implementation of active contours in 2D as well as in 3D. I will also explore the choice of the parameters with a quantitative study of their influence on the segmentation results.
Active contours have shown their ability to introduce regularity on binary image segmentation. In contrast with level-set approaches, they are able to maintain the initial topology of the area of interest. For this reason, it has been used in recent medical research for diaphragm segmentation. Most of the online codes for 3D segmentation, as well as built-in Matlab toolboxes, are based on level-set methods. Moreover, in the literature, the implementation details of active contours methods with meshes in three dimensions are tight, making tedious any reproduction of these techniques. In this talk, I will present some details on the implementation of active contours in 2D as well as in 3D. I will also explore the choice of the parameters with a quantitative study of their influence on the segmentation results.
Sebastian Baudelet, Université of Lorraine
Apr 29th 2020, 6pm, videoconference from Boston
Modelling of the Mitral Valve Closure in 2D
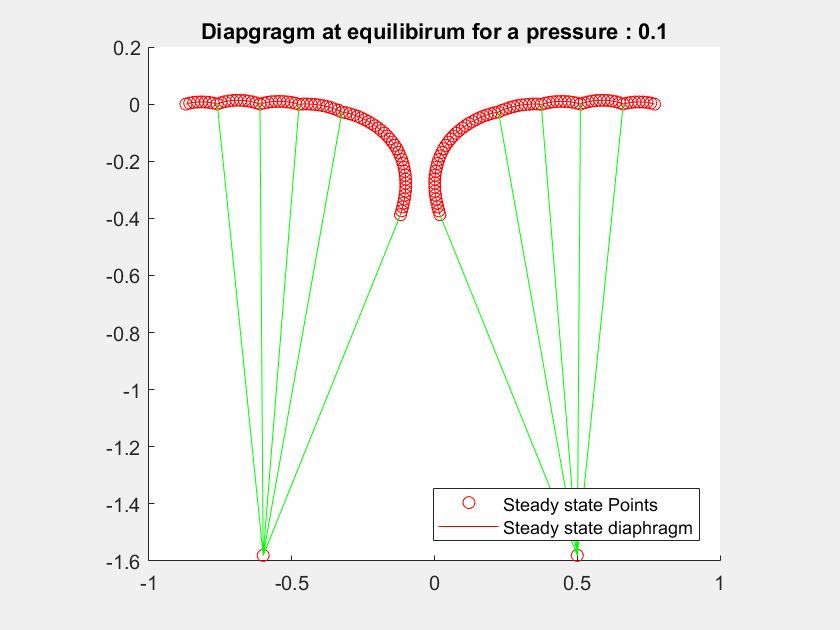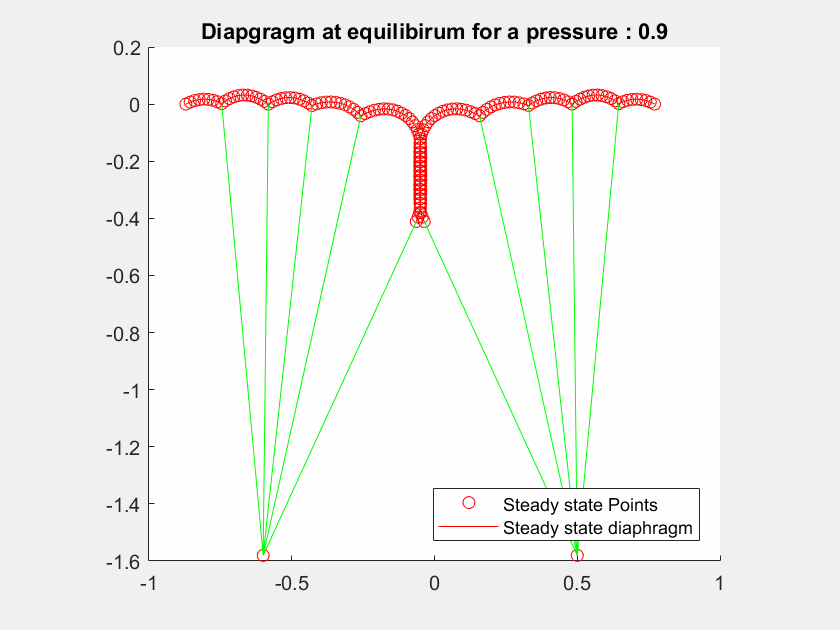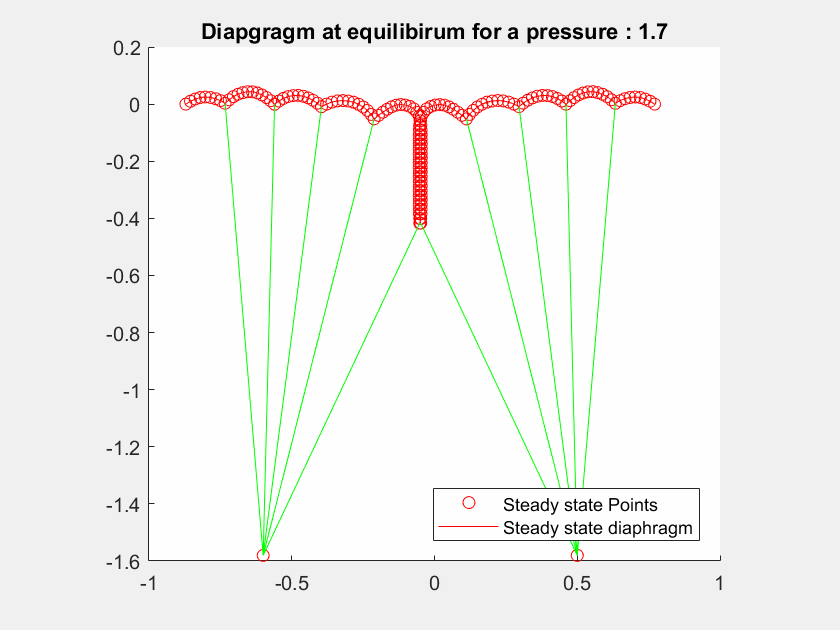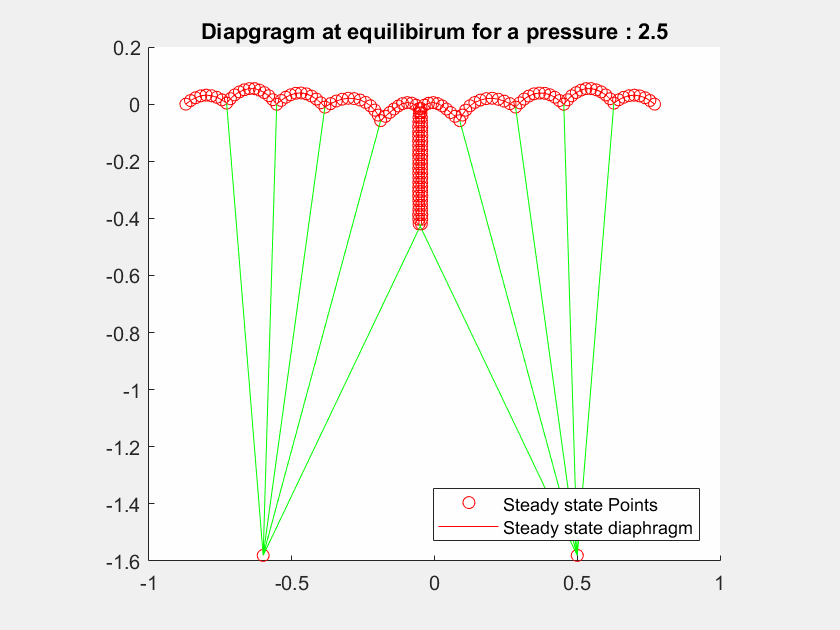 The talk will present a strategy to model the mitral valve, which is an elastic material, by a mass-spring system, modelling both the leaflets and the chordae tendineae with different constant stiffness extracted from the literature.
The talk will present a strategy to model the mitral valve, which is an elastic material, by a mass-spring system, modelling both the leaflets and the chordae tendineae with different constant stiffness extracted from the literature.
The valve geometry during peak systole is a quasi-static equilibrium in which its configuration can be computed. I will present here a method to evaluate the valve positions in a 2D case by maximizing the Lagrangian function.
I will first present a mathematical formalization of the problem, and then a numerical method to optimize the computation of the deformed geometry to achieve interactive-time simulation.
The method has been tested on different geometries with various boundary condition cases and includes blood pressure and contact forces between leaflets.
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
Harvard University, Dec 3rd 2019, 12:30pm Room Seminar room (311) 60 Oxford street
An Overview of Deformable Models
 This talk presents an overview of deformable models that I have used during my research work. I have gathered them into three groups: i) Fully geometry-based, ii) Partially based on Physics and iii) Fully based on physics.
This talk presents an overview of deformable models that I have used during my research work. I have gathered them into three groups: i) Fully geometry-based, ii) Partially based on Physics and iii) Fully based on physics.
In the first group, I will describe the chainmail algorithm and
free-form deformations. I will show applications for modeling the respiration. In the the second group, I will present mass-spring system and the Cosserat model. Applications include inguinal hernia repair and diaphragm modeling. Eventually in the last group, I will overview the work I have done using shell FEM elements, volume FEM elements and my current work on Lagrangian minimization and Radial Basis Functions with various applications.
Daryna Panicheva, Université of Lorraine – Loria – Inria
Harvard University, Nov 19th 2019, 12:30pm, Room Seminar room (311) 60 Oxford street
Physically-coherent Extraction of Mitral Valve Chordae
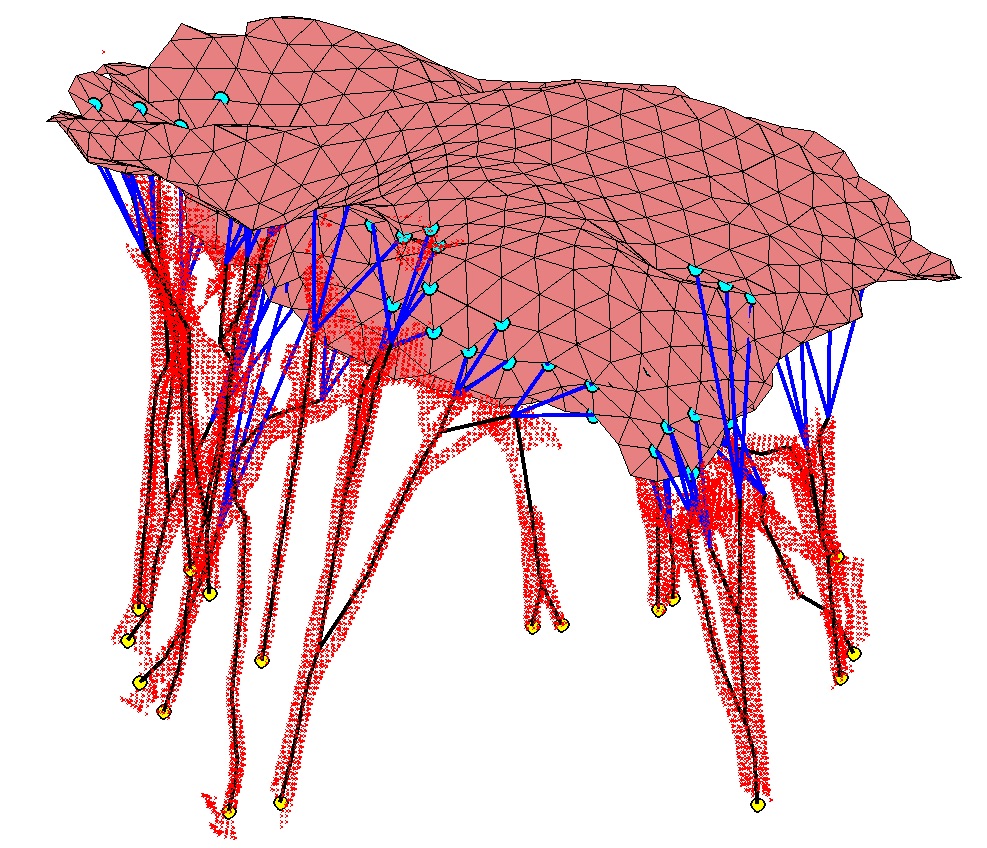 Mitral valve dysfunction is one of the common cardiovascular diseases. Repair outcome can be significantly improved with an image-based computer simulation tool. In order to avoid tedious manual segmentation of the valve components, we propose an approach that allows automatic extraction of the valve chordae as a step to the valve behavior modeling. The method is based on the topology analysis of the structures and requires only maximum radius of the chordae as a parameter. Proposed approach was tested on three porcine datasets and it was shown to correctly detect main structures of the chord.
Mitral valve dysfunction is one of the common cardiovascular diseases. Repair outcome can be significantly improved with an image-based computer simulation tool. In order to avoid tedious manual segmentation of the valve components, we propose an approach that allows automatic extraction of the valve chordae as a step to the valve behavior modeling. The method is based on the topology analysis of the structures and requires only maximum radius of the chordae as a parameter. Proposed approach was tested on three porcine datasets and it was shown to correctly detect main structures of the chord.
Douglas Perrin, Harvard Biorobotics Lab
Inria Grand Est, September 25th 2019, 10am Room C103
Patient-Specific Mitral Valve Segmentation from 4D Ultrasound.
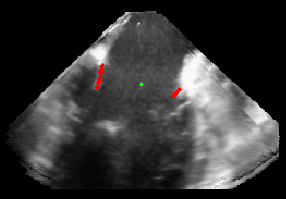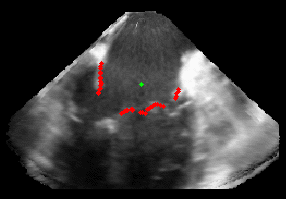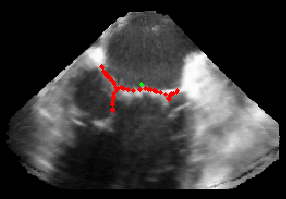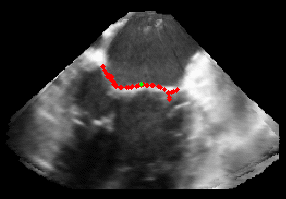 Successful clinical treatment of mitral valve disease is dependent on understanding the complexities of patient-specific valve behavior. Mechanical models of the mitral valve, designed to predict valve closure and generated using patient-specific measurements, have proven to be useful tools for studying valve behavior. To be clinically feasible, these models need to come from ultrasound images.
Successful clinical treatment of mitral valve disease is dependent on understanding the complexities of patient-specific valve behavior. Mechanical models of the mitral valve, designed to predict valve closure and generated using patient-specific measurements, have proven to be useful tools for studying valve behavior. To be clinically feasible, these models need to come from ultrasound images.
I will be presenting work done in collaboration with Dr. Rob Schneider during his PhD thesis. In this work, we developed a method for generating patient-specific mitral valve model (annulus, leaflets, state) from three-dimensional ultrasound. I will describe our automated approach for initial demarcation of the mitral valve annulus, which we compared to the results of manual delineations made by experts. Using a valve state predictor, we developed and a constrained optical flow algorithm we segment the annulus in the remaining frames in the ultrasound sequence. The location of the leaflets in the image-frame just before valve closure is then automatically found by using the previously delineated annulus to bound the segmentation. Using active surface model, the leaflet geometry, and four-dimensional (4D) annulus the leaflets are tracked during valve closure. This enabled, for the first time, the segmentation of an accurate and detailed mitral leaflet coaptation region.
Peter E. Hammer, Harvard Biorobotics Lab
Inria Grand Est, July 1st 2019, 2pm Room C103
Computational fluid dynamics to guide care of patients with congenital heart disease.
 Computational fluid dynamics (CFD) refers to use of computers to numerically approximate the Navier-Stokes equations, which are the basic conservation laws that govern fluid flow. This methodology is well established in the design of mechanical systems and has more recently been used to study biological flows in the human body. In this talk, I will describe recent work by my group using CFD to better understand blood flow in patients with various forms of congenital heart disease, including single-ventricle defects, anomalous coronary arteries, and pulmonary vein stenosis. I will give examples of both disease-specific studies, where the goal is generalized treatment guidelines, and patient-specific CFD studies, where results can directly inform patient care.
Computational fluid dynamics (CFD) refers to use of computers to numerically approximate the Navier-Stokes equations, which are the basic conservation laws that govern fluid flow. This methodology is well established in the design of mechanical systems and has more recently been used to study biological flows in the human body. In this talk, I will describe recent work by my group using CFD to better understand blood flow in patients with various forms of congenital heart disease, including single-ventricle defects, anomalous coronary arteries, and pulmonary vein stenosis. I will give examples of both disease-specific studies, where the goal is generalized treatment guidelines, and patient-specific CFD studies, where results can directly inform patient care.
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
Harvard University, Nov 26th 2018, 4:45pm Room Seminar room (311) 60 Oxford street
Mitral Valve modeling: from CT scan FEM model
 Common surgical procedures on mitral heart valves include chordae structure modifications. Such interventions will happen when there is an extensive prolapse of a leaflet caused by chordae rupture or chordae elongation. Understanding and simulating the importance of chordae tendineae before operating could be helpful in order to anticipate if the mitral valve will maintain a consistent closure shape during the systolic peak. Biomechanical modeling and simulation can achieve such a goal.
Common surgical procedures on mitral heart valves include chordae structure modifications. Such interventions will happen when there is an extensive prolapse of a leaflet caused by chordae rupture or chordae elongation. Understanding and simulating the importance of chordae tendineae before operating could be helpful in order to anticipate if the mitral valve will maintain a consistent closure shape during the systolic peak. Biomechanical modeling and simulation can achieve such a goal.
I will present a method to semi-automatically build a mitral valve biomechanical model from micro CT scan: after manually picking chordae fiducial points, the leaflets are segmented and the boundary conditions, as well as the loading conditions, are automatically defined. Fast FEM simulation is carried out using SOFA (Simulation Open Framework Architecture) to reproduce leaflets closing during systolic peak. We validate our method on three explanted porcine hearts and show that our model performs well: point-to-surface error around 1mm, reasonably large coaptation surface and no leak at the systolic peak.
Daryna Panicheva, Université of Lorraine – Loria – Inria
Harvard University, Nov 19th 2018, 3pm lecture hall (330) 60 Oxford street
Automatic Segmentation of Mitral Valve Chordae
 Mitral valve dysfunction is one of the common cardiovascular diseases. Repair outcome can be significantly improved with an image-based computer simulation tool. In order to avoid tedious manual segmentation of the valve components, we propose an approach that allows automatic extraction of the valve chordae as a step to the valve behavior modeling. The method is based on the topology analysis of the structures and requires only maximum radius of the chordae as a parameter. Proposed approach was tested on three porcine datasets and it was shown to correctly detect main structures of the chord.
Mitral valve dysfunction is one of the common cardiovascular diseases. Repair outcome can be significantly improved with an image-based computer simulation tool. In order to avoid tedious manual segmentation of the valve components, we propose an approach that allows automatic extraction of the valve chordae as a step to the valve behavior modeling. The method is based on the topology analysis of the structures and requires only maximum radius of the chordae as a parameter. Proposed approach was tested on three porcine datasets and it was shown to correctly detect main structures of the chord.
Robert D. Howe, Harvard Biorobotics Lab
Inria Grand Est, June 18th 2018, 9am Room C103
Fixing the Beating Heart: Ultrasound Guidance for Robotic Intracar diac Surgery.
diac Surgery.
To treat defects within the heart, surgeons currently use stopped-heart techniques. These procedures are highly invasive and incur a significant risk of neurological impairment. We are developing methods for performing surgery within the heart while it is beating. New real-time 3-D ultrasound imaging allows visualization through the opaque blood pool, but this imaging modality poses difficult image processing challenges due to poor resolution, acoustic artifacts, and data rates of 30 to 40 million voxels per second. To track instruments within the heart we have developed a Radon transform-based algorithm. Implementation using a graphics processor unit (GPU) enables real-time processing of the ultrasound data stream. For manipulation of rapidly moving cardiac tissue we have created a fast robotic device that can track the tissue based on ultrasound image features. This allows the surgeon to interact with the heart as if it was stationary. Our in vitro studies show that this approach enhances dexterity and lowers applied forces. To complete integration of ultrasound imaging with the robotic device we have developed a predictive controller that compensates for the imaging and image processing delays to ensure good tracking performance. We will present applications of this technology in atrial septal defect closure and mitral valve annuloplasty procedures, demonstrating the potential for improved patient outcomes.
Peter E. Hammer, Harvard Biorobotics Lab
Inria Grand Est, June 6th 2018, 3pm Room A006
Using engineering and computational methods to inform surgery for congenital heart disease.
 Surgeons have traditionally used an approach based on clinical experience and intuition. However, in congenital heart disease, the variety of anatomical abnormality is almost endless, defying an experience-based approach. Analytical approaches based on quantitative analysis and engineering principles have been proposed and show promise to help improve surgical management in highly complex cases. In this talk, examples will be presented to show the use of quantitative approaches to inform surgery, including: (1) characterization of the mechanical properties of cardiovascular tissues and use of this data to guide surgical techniques, (2) simulation to explore novel methods for valve reconstruction in the growing child, (3) lumped element modeling of the circulation to aid in decision-making for complex circulations.
Surgeons have traditionally used an approach based on clinical experience and intuition. However, in congenital heart disease, the variety of anatomical abnormality is almost endless, defying an experience-based approach. Analytical approaches based on quantitative analysis and engineering principles have been proposed and show promise to help improve surgical management in highly complex cases. In this talk, examples will be presented to show the use of quantitative approaches to inform surgery, including: (1) characterization of the mechanical properties of cardiovascular tissues and use of this data to guide surgical techniques, (2) simulation to explore novel methods for valve reconstruction in the growing child, (3) lumped element modeling of the circulation to aid in decision-making for complex circulations.
Pierre-Frederic Villard, Université of Lorraine – Loria – Inria
Harvard University, Oct 31th 2017, 1pm Room Seminar room (311) 60 Oxford street
Automatic Reconstruction of Mitral Valve Chordae
 Mitral valve disorders are one of the most common cardiac diseases. The treatment of such pathologies often requires surgery.
Mitral valve disorders are one of the most common cardiac diseases. The treatment of such pathologies often requires surgery.
To help the doctor to choose the surgical procedure and to be able to anticipate the result of the repair, the biomechanical modeling of the valve is done. The existing models are rather simplistic and generic, which is why we are interested in models specific to the patient. More precisely, models based on CT scans of heart which components are segmented and thereafter used in order to define the mechanical characteristics of the valve.
In this work, we focused on the segmentation of the valve cords. As dataset, we used 8 CT images of porcine hearts. The proposed solution consists of applying the RANSAC-like method with the image contours as a set of points. The RANSAC methods extract points corresponding to the parametric model (in our case cylindrical). To limit the area of research we proposed to use as initial assumptions of cords location the results of segmentation with classical methods for tubular structures extraction.
The proposed method allows to significantly improve cords segmentation results compared with classical methods, in particular, the section size and the endpoints of the cords are defined which is important for following mechanical modeling. Future work involves the treatment of difficult cases (presence of bifurcations) and validation of the results that have been made only visually in this work.
Thomas Waite, Harvard Biorobotics Lab
Inria Grand Est, June 6th 2017, 2pm Room C103
Cosserat Rods for Modeling Robotic Catheter Systems
 Tendon-driven robotic catheters are capable of precise execution of minimally invasive cardiac procedures including ablations and imaging. However, these procedures require accurate modeling of not only the catheter and tendons but also their interactions with surrounding tissue and vasculature. For this reason, mechanically-derived models provide a clear advantage over geometric models, given the ease with which mechanical models handle contact forces and friction. As a solution, we present a fully-mechanical model of a tendon-driven robotic catheter system based on Cosserat rods and integrated with a stable, implicit scheme. We first validate the physical accuracy of the Cosserat rod as a model for a simple catheter centerline against an analytical model for large deformations and experimental data. We then expand the system by adding a second Cosserat rod to model a single tendon in addition to the catheter and define the constraints of the tendon-catheter system with penalty forces. We then validate the resulting tendon-catheter system against experimental data to prove its physical accuracy. This model represents a new contribution to the field of robotic catheter modeling in which both the tendons and catheter are modeled by fully-mechanical Cosserat rods and fully validated against experimental data.
Tendon-driven robotic catheters are capable of precise execution of minimally invasive cardiac procedures including ablations and imaging. However, these procedures require accurate modeling of not only the catheter and tendons but also their interactions with surrounding tissue and vasculature. For this reason, mechanically-derived models provide a clear advantage over geometric models, given the ease with which mechanical models handle contact forces and friction. As a solution, we present a fully-mechanical model of a tendon-driven robotic catheter system based on Cosserat rods and integrated with a stable, implicit scheme. We first validate the physical accuracy of the Cosserat rod as a model for a simple catheter centerline against an analytical model for large deformations and experimental data. We then expand the system by adding a second Cosserat rod to model a single tendon in addition to the catheter and define the constraints of the tendon-catheter system with penalty forces. We then validate the resulting tendon-catheter system against experimental data to prove its physical accuracy. This model represents a new contribution to the field of robotic catheter modeling in which both the tendons and catheter are modeled by fully-mechanical Cosserat rods and fully validated against experimental data.
Douglas Perrin, Harvard Biorobotics Lab
Inria Grand Est, May 30th 2017, 2pm Room C103
Machine Learning and Image Processing: from the Lab to Clinical Use
In this presentation he talked about his work in the Cardiac Surgery Department at Boston Children’s Hospital on translating recent results in machine learning and image processing from the lab into clinical use. The approach has been to build collaborative teams consisting of medical fellows, surgical and cardiology attending physicians, industrial collaborators, and faculty from the Harvard School of Engineering and Applied Sciences along with their Ph.D. students and post-docs. He talked about some of the benefits and pitfalls of this approach. Specifically, challenges surrounding getting data within a hospital environment as well as lessons learned on project integration across clinical and engineering expertise. He went into detail on one project where we have been using machine learning to inspect echo-cardiograms for congenital heart defects. Ultrasound images are quite different from natural images, for which artificial neural networks have already been successfully applied to for classification tasks. This imaging modality has a more challenging noise profile than photography images and may not have features directly linked to the specific class (i.e. the pathology). That said they had success extracting intrinsic characteristics (view) from these types of images and their classification results are more accurate than simple random chance for several of the pathologies of interest. They believe this work represents an important step in developing automated screening tools for these diseases. In the case of birthing centers without pediatric cardiologist on site, these methods represent an effective way to transfer expertise from larger centers that will have a direct impact on the number of newborns with these pathologies that can be detected and treated.
talked about his work in the Cardiac Surgery Department at Boston Children’s Hospital on translating recent results in machine learning and image processing from the lab into clinical use. The approach has been to build collaborative teams consisting of medical fellows, surgical and cardiology attending physicians, industrial collaborators, and faculty from the Harvard School of Engineering and Applied Sciences along with their Ph.D. students and post-docs. He talked about some of the benefits and pitfalls of this approach. Specifically, challenges surrounding getting data within a hospital environment as well as lessons learned on project integration across clinical and engineering expertise. He went into detail on one project where we have been using machine learning to inspect echo-cardiograms for congenital heart defects. Ultrasound images are quite different from natural images, for which artificial neural networks have already been successfully applied to for classification tasks. This imaging modality has a more challenging noise profile than photography images and may not have features directly linked to the specific class (i.e. the pathology). That said they had success extracting intrinsic characteristics (view) from these types of images and their classification results are more accurate than simple random chance for several of the pathologies of interest. They believe this work represents an important step in developing automated screening tools for these diseases. In the case of birthing centers without pediatric cardiologist on site, these methods represent an effective way to transfer expertise from larger centers that will have a direct impact on the number of newborns with these pathologies that can be detected and treated.