PIONeeR – QUANTIC – QUANTitative modeling combined to statistical learning to understand and predict resistance to Immune-checkpoint inhibition in non-small cell lung Cancer
P.I. : S. Benzekry
COMPO members involved : L Greillier, J. Ciccolini, P. Dufossé, C. Bigarre, S. Marolleau, M. Boussena
Partners : Clinicians: P. Tomasini (AP-HM), F. Barlesi (IGR), Institutional: AP-HM, Inserm, CNRS, Marseille Immunopole, CIML. Researchers: D. Olive (CRCM), E. Vivier/F. Vély (CIML), F. Sabatier (AP-HM), Industrials: Halio DX/Veracyte, Innate Pharma.
Clinical need
The rise of Immune Checkpoint Inhibitors (ICI) has provided significant improvement in survival rates for advanced NSCLC, but only 20-30% of the patients treated with ICIs exhibit a long-term survival benefit. There is a lack of validated biomarkers and integrative multi-modal analyses to predict response and resistance.
Objective
The primary objective of QUANTIC is to develop and validate combined mechanistic mathematical models with machine learning, which are predictive of response and toxicity to ICI from baseline and early longitudinal data gathered from the PIONeeR project.
Data
QUANTIC relies on modeling unique, large scale (n = 450 patients), heterogeneous and longitudinal data collected during the PIONeeR (“Precision Immuno-Oncology for advanced Non-small cell lung cancer patients with PD-(L)1 ICI Resistance”) biomarkers clinical study.
Methodology
1. build mathematical models able to simulate the kinetics of response to treatments on the basis of biological hypotheses,
2. design adequate statistical methods able to identify parameters from the models using large-dimension data
3. establish patient-specific predictive models.
Funding
- Inserm National Cancer Institute Plan Cancer MIC (Mathématiques et Informatique pour le Cancer), 2019 – 2022
- National Research Agency RHU PIONeeR, 2018 – 2024
- Laënnec Institute, AMU
Updated news on the Pioneer Project
Publications
Comprehensive biomarkers analysis to predict resistances to PD1/L1 ICIs in advanced Non-small cell lung cancer patients: results of the Precision Immuno-Oncology for advanced Non-Small CEll Lung CancER (PIONeeR) biomarkers profiling study
Greillier, F. Monville, …, M. Karlsen, P. Dufosse, M. Boussena, A. Vaglio. ,…, S. Benzekry, F. Barlesi
in preparation, 2024
Comprehensive biomarkers analysis to predict resistances to PD1/L1 ICIs in advanced Non-small cell lung cancer patients: rationale and protocol of the Precision Immuno-Oncology for advanced Non-Small CEll Lung CancER (PIONeeR) biomarkers profiling study
L. Greillier L, M. Roumieux, F. Monville, … A. Vaglio, …, S. Benzekry, F. Barlesi
in preparation, 2024
Feature selection is not a stable journey: a benchmark on a complex biomedical dataset in the p ~ n case
A. Bakhmach, S. Benzekry
in preparation, 2024
Mechanistic Learning for Predicting Survival Outcomes in Head and Neck Squamous Cell Carcinoma
K. Atsou, A. Auperin, J. Guigay, S. Salas, S. Benzekry
under review, hal, 2024
Predicting survival in patients with advanced NSCLC treated with atezolizumab using pre- and on-treatment prognostic biomarkers
S. Benzekry, M. Karlsen, C. Bigarré, A. El Kaoutari, B. Gomes, M. Stern, A. Neubert, R. Bruno, F. Mercier, S. Vatakuti, P. Curle and Candice Jamois.
Clinical Pharmacology and Therapeutics, 116, 1110–112, 2024
Machine learning for prediction of immunotherapy efficacy in non-small cell lung cancer from simple clinical and biological data
Benzekry, M. Grangeon, M. Karlsen, M. Alexa, I. Bicalho-Frazeto, S. Chaleat, P. Tomasini, D. Barbolosi, F. Barlesi, L. Greillier
Cancers, Volume 5, pp. 81-90, medRxiv, 2021
Artificial intelligence and mechanistic modeling for clinical decision making in oncology
S. Benzekry
Clinical Pharmacology and Therapeutics, Volume 108, Issue 3, pp.471-486, 2020
Deciphering the response to immune-checkpoint inhibitors in lung cancer with artificial intelligence-based analysis: the pioneer and quantic joint-projects.
J. Ciccolini, S. Benzekry, F. Barlesi.
British Journal of Cancer, 2020, 123 (3)
Communications
Une comparaison des algorithmes d’apprentissage pour la survie avec données manquantes
P. Dufossé, S. Benzekry
SFdS, arXiv, 2023
Comprehensive biomarkers (BMs) analysis to predict efficacy of PD1/L1 immune checkpoint inhibitors (ICIs) in combination with chemotherapy: a subgroup analysis of the Precision Immuno-Oncology for advanced Non-Small CEll Lung CancER (PIONeeR) trial.
F. Barlesi, L. Greillier, …, J. Ciccolini, M. Karlsen, P. Dufossé,…, S. Benzekry
ESMO IO, Abstr 3MO, 2022
September, 2024. Twelfth International Workshop on Pharmacodynamics of Anticancer Agents. “Use of machine learning for predicting cancer outcomes”, Ponta Delgada, Portugal.
S. Benzekry
July, 2024. ECMTB congress (European Conference on Mathematical and Theoretical Biology). “Integrating kinetics and machine learning modeling for prediction of outcome following immunotherapy in lung cancer”, talk within the mini-symposium “Digital twins for clinical oncology and cancer research” organized by G. Lorenzo, Toledo, Spain.
S. Benzekry
July, 2024. SMB congress (Society of Mathematical Biology). “Integrative kinetics and machine learning modeling for prediction of outcome following immunotherapy in lung cancer”. Talk within the mini-symposium “Mathematical modeling of cancer treatment” organized by E. Kim, Seoul, Korea.
S. Benzekry
June 2024, PAGE, Roma, Italy. Mechanistic modelling of tumor kinetics coupled with biomarker dynamics for survival prediction in non-small cell lung cancer patients
R. Taieb, R. Bruno, J. Jin, P. Chanu, S. Benzekry
June, 2024. BIOMAT summer school. Mini-course “Mechanistic learning to predict response and survival in immuno-oncology.”, Granada, Spain.
S. Benzekry
March, 2024. Keynote invited speaker in the session “Machine Learning for Rare Disease Applications: from Limited to In Silico Trials” of the Applied machine learning days (AMLD2024), “Mechanistic learning to predict the results of clinical trials”, EPFL, Lausanne.
S. Benzekry
January, 2024. EORTC-PAMM, The PIONeeR trial, Marseille.
S. Benzekry
November 2023, SITC, Paris, France. Regulatory T-cell tumor infiltration improves advanced NSCLC patients’ outcome under ≥ 2nd line anti-PD1/L1 monotherapy in The PIONeeR Project. Poster
M. Landri, …, S. Benzekry, …, J. Fieschi
Workshop for young researchers in Cancer.” Integrative kinetics and machine learning modeling for prediction of outcome following immunotherapy in lung cancer“.
Cancéropôle PACA, Porquerolles, 2023
S. Benzekry (keynote speaker)
Challenges in data-driven biomarkers research
P. Dufossé
TRANSLATE-IT symposium, 2023, Marseille
June, 2023. Annual congress of the French group of clinical pneumo-oncology (GFPC). ” Artificial intelligence in healthcare data warehouses, Toulon, France.
S. Benzekry
June, 2023. Annual congress of the French society of pharmacology and therapeutics (SFPT), Integrative kinetics and machine learning modeling for prediction of outcome following immunotherapy in lung cancer, Limoges, France.
S. Benzekry
May, 2023. SMAI congress (French Society of Applied and Industrial Mathematics). Talk within the mini-symposium “Modélisation Mathématique du cancer et de son environnement” organized by F. Hubert and M. Mezache. Pointe à Pitre, France
S. Benzekry
March, 2022. MI Day ” Immunotherapies & Lung Cancer : predictive biomarkers from The PIONeeR Project “, Marseille, France
S. Benzekry
Comprehensive biomarkers analysis to explain resistances to PD1-L1 ICIs: The precision immuno-oncology for advanced non-small cell lung cancer (PIONeeR) trial.
L. Greillier, …, J. Ciccolini, M. Karlsen, P. Fleury, … S. Benzekry, J. Fieschi, F. Barlesi.
AACR, Abstr LB120, Cancer Res, 82 (12_Supplement): LB120, 2022
Supporting decision making and early prediction of survival for oncology drug development using a pharmacometrics-machine learning based model
Benzekry, M. Karlsen, A. El Kaoutari, R. Bruno, A. Neubert, F. Mercier, M. Stern, B. Gomes, S. Vatakuti, P. Curle and Candice Jamois.
PAGE 30, Abstr 10276, 2022
_________________________________________________________________________________________________________________________
Mechanistic modeling for the prediction of metastatic relapse in breast cancer
P.I.s : S. Benzekry, X. Muracciole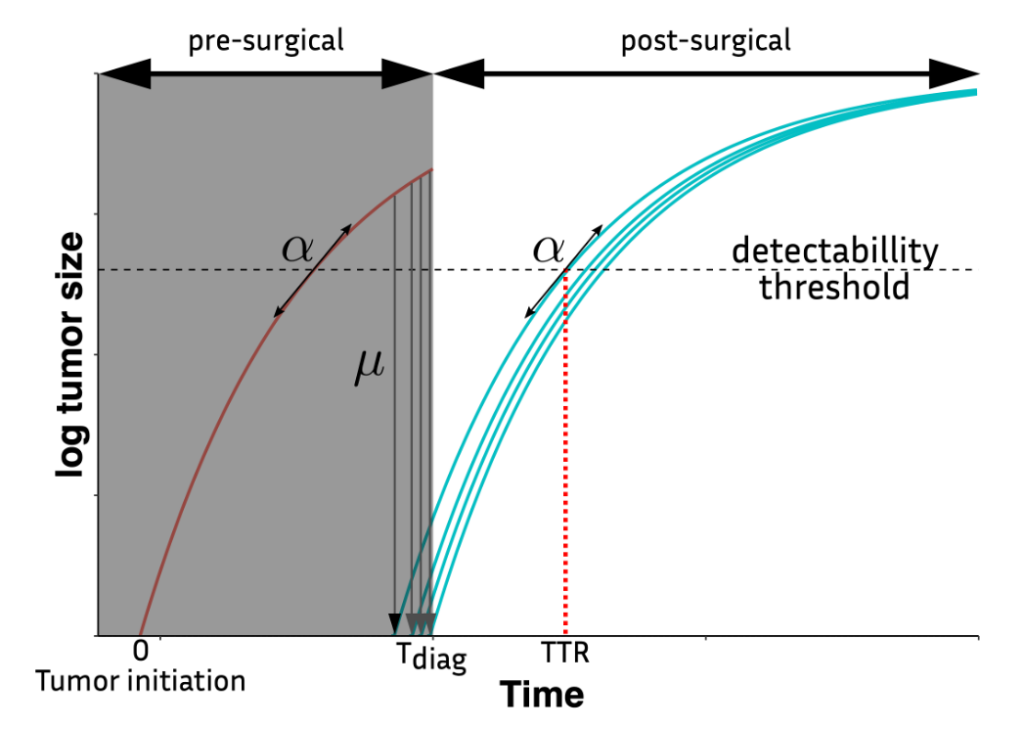
COMPO members involved : C. Bigarré, D. Barbolosi
Partners : G. MacGrogan (Bergonié Institute Bordeaux), F. Bertucci (Institut Paoli-Calmettes, CRCM)
Clinical need :
Estimation of the risk of metastatic relapse is a major challenge to decide treatment options for early-stage breast cancer patients.
Objective
The objective of this work is to use clinical data to calibrate a mechanistic model to predict metastatic relapse algorithms.
Data
We have access to clinical data from breast cancer patients from the Bergonié Institute (N = 1161 patients), the APHM radiotherapy department (N = 167) and several public databases with tumor genomic information aggregated by our partners at IPC (N = 992).
Methodology
To date, metastatic free survival (MFS) analysis mainly relies on classical – agnostic – statistical models (e.g., Cox regression). Instead, we propose to derive mechanistic models to predict MFS. We use the statistical framework of mixed-effects models to describe the variability of the parameters of the model in the population.
Publications:
Development and Validation of a Prediction Model of Overall Survival in High-Risk Neuroblastoma Using Mechanistic Modeling of Metastasis
S. Benzekry, C. Sentis, C. Coze, L. Tessonnier, N. André
JCO: Clin Cancer Informatics, Volume 5, pp. 81-90, medRxiv, 2021
Machine learning and mechanistic modeling for prediction of metastatic relapse in breast cancer
C. Nicolo, C. Perier, M. Prague, G. MacGrogan, O. Saut, S. Benzekry
JCO: Clinical Cancer Informatics, Volume 4, pp. 259-274, bioRxiv, 2020
Modeling spontaneous metastasis following surgery: an in vivo-in silico approach
S. Benzekry, A. Tracz, M. Mastri, R. Corbelli, D. Barbolosi, J.M.L. Ebos
Cancer Research, Volume 76, Issue 3, pp. 535-547, hal, 2016
_________________________________________________________________________________________________________________________
SChISM – Size CfDNA Immunotherapies Signature Monitoring
P.I.s : S. Benzekry, S. Salas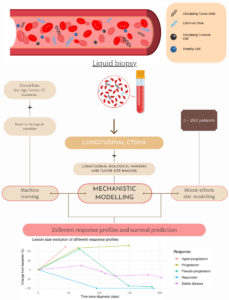
COMPO members involved : L. Nguyen Phuong
Partners : M. Lavielle (XPOP – Inria), Adelis, F. Fina (Adelis, ID-Solutions)
Clinical need
A major immuno-oncology issue is currently to early predict the response of
immunotherapy for cancer patients, ideally before the first imaging exam. Circulating DNA available from liquid biopsies are a promising, non-invasive, surrogate to imaging to early assess response during treatment.
Objective
The objective is to observe and model real-time variations in concentration and size of cell-free plasma DNA (cfDNA) to early predict response and survival following immunotherapy for five cancer types (melanoma, metastatic head and neck squamous-cell carcinoma, clear cell kidney cancer, urothelial bladder carcinoma and non-small cell lung cancer), using a novel, patented, standardized and innovative technology provided by our partners, ID-solutions and Adelis.
Data
We are collecting data on 260 patients starting an anti-PDL1/PD1 treatment (possibly adjuvant). These consist of baseline and longitudinal data, the latter beinglongitudinal data from standardized ID-Solutions/ADELIS extraction, corresponding to concentration and size of cfDNA fragments at different timepoints, biological (hematology and biochemistry) and imaging data.
Methodology
We are developing a mechanistic model to describe kinetics of cfDNA. Statistical nonlinear mixed-effects modeling is used to quantify inter-patient variability. Eventually, statistical machine learning methods are finally used to build predictive algorithms.
Publications:
Computational modeling approaches for circulating cell-free DNA in oncology
L. Nguyen Phuong, S. Salas, S. Benzekry
preprint, hal.science, 2024
Communications:
Quantitative cell-free DNA markers for prediction of early progression in patients undergoing immunotherapy
L. Nguyen Phuong, L. Greillier, C. Gaudy-Marqueste, J.-L. Deville, J.-C. Garcia, F. Fina, S. Salas, S. Benzekry
Summer School AI4Health, Paris, France, 2023
Annnual meeting of the Institute of Cancer and Immunologie of Marseille, Marseille, France, 2023
Mechanistic modeling of the longitudinal tumor and biological markers combined with quantitative cell-free DNA
L. Nguyen Phuong, L. Greillier, C. Gaudy-Marqueste, J.-L. Deville, J.-C. Garcia, F. Fina, S. Salas, S. Benzekry
Annual Meeting of the Society for Mathematical Biology (SMB), Columbus (Ohio), USA, 2023
Circulating cell-free DNA size distribution as a prediction marker for early progression undergoing immune checkpoint inhibitors
L. Nguyen Phuong, F. Fina, L. Greillier, C. Gaudy-Marqueste, J.-L. Deville, J.-C. Garcia, S. Salas, S. Benzekry
Annual Meeting of the Population Approach Group Europe (PAGE), Rome, 2024
Long cell-free DNA fragments predict early-progression in patients with advanced or metastatic cancer treated with immune-checkpoint inhibition
S. Salas, L. Nguyen Phuong, J.-C. Garcia, L. Greillier, C. Gaudy-Marqueste, A. Boutonnet, F. Ginot, S. Benzekry
Annual meeting of the American Society for Clinical Oncology, Chicago (Illinois), USA, 2024
Integrating tumor kinetics and circulating cell-free DNA markers through mechanistic modeling for early prediction of immunotherapy response
L. Nguyen Phuong, F. Fina, L. Greillier, C. Gaudy-Marqueste, J.-L. Deville, J.-C. Garcia, S. Salas, S. Benzekry
Annual Meeting of the KSMB-SMB, Seoul, 2024
Long circulating-free DNA fragments predict early-progression (EP) and progression-free survival (PFS) in advanced carcinoma treated with immune-checkpoint inhibition (ICI): a new biomarker
S. Salas, L. Nguyen Phuong, F. Ginot, L. Greillier, P. Tomasini, A. Boutonnet, J.-L. Deville, F. Fina, S.Benzekry
Annual meeting of the European Society for Medical Oncology, Barcelona, Spain, 2024
_________________________________________________________________________________________________________________________
Machine learning for prediction of response to immunotherapy using real world data
P.I.s S. Benzekry, L. Greiller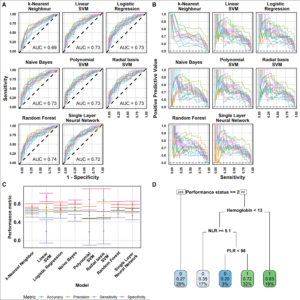
COMPO members involved D. Barbolosi, M. Karlsen, P. Fleury
Partners F. Barlesi (Gustave Roussy Institute)
Clinical need
Immune checkpoint inhibitors (ICIs) are now a therapeutic standard in advanced non-small cell lung cancer (NSCLC), but only 20% of patients do respond and strong predictive markers for efficacy are lacking.
Objective
Leverage clinical and biological data available from routine measurements to predict response and toxicity to ICIs using machine learning (ML) algorithms, in order to individually adapt therapeutic interventions.
Data
So far, we analyzed data of 298 second-line (or more) stage IV non-small cell lung cancer (NSCLC) patients, featuring 10 pre-treatment blood count variables together with clinical variables.
Methodology
We use data science techniques to study the association of these routine data with response, progression free survival and overall survival. The general supervised learning method consists of two main steps: feature selection and statistical learning using ML.
Publications:
Machine learning for prediction of immunotherapy efficacy in non-small cell lung cancer from simple clinical and biological data
S. Benzekry, M. Grangeon, M. Karlsen, M. Alexa, I. Bicalho-Frazeto, S. Chaleat, P. Tomasini, D. Barbolosi, F. Barlesi, L. Greillier
Cancers, Volume 5, pp. 81-90, medRxiv, 2021
Artificial intelligence and mechanistic modeling for clinical decision making in oncology
S. Benzekry
Clinical Pharmacology and Therapeutics, Volume 108, Issue 3, pp.471-486, 2020


