CETUXIMAX:
Cetuximab Therapeutic Drug Monitoring in Squamous Cell Carcinoma Head and Neck Cancer Patients: Determination of the Predictive Value Exposure Levels Through a Single Arm Multicentric Study (CETUXIMAX)
P.I.: S. Salas (COMPO)
COMPO members involved: J. Ciccolini, M. Hamimed, C. Marin
Partners: Merck (Sponsor), APHM (promoter), CHU Timone, + 15 investigating centers
Clinical need:
Anti-EGFR cetuximab is a pivotal biotherapy for treating head and neck cancer patients. However, only 50% of response rate have been reported. Determining a trough level associated with target engagement and clinical efficacy would pave the way for PK-guided dosing.
Objectives:
To study the PK/PD relationships of cetuximab in head and neck cancer patients.
Data
PK data will be generated upon blood sampling (Cmin, cmax) in a longitidinal fashion accrossthe different courses. Clinical data (efficacy, toxicity per CTCAE grading) will be similarly collected in a longitudinal fashion.
Methodology,
1. Cetuximax is a multicentric study registered as NCT04218136.
2. Cetuximab will be assayed for plasma concentrations by a fully validated LC-MS/MS method.
3. Individual PK parameters will be estimated using a Bayesian procedure and pop-PK matrix using a compartmental approach. Parameters identification (Kel, Vd, Cl, T1/2) and estimation of exposure metrics (AUC) will be performed on Monolix.
4. PK/PD relationships will be investigated on Monolix software.
Funding
Merck Serono.
BUSEQ:
PK-guided dosing of High dose Busulfan as part of conditioning regimen in adult leukemia patients scheduled for bone marrox transplant.
P.I.: S. Furst (IPC)
COMPO members involved: J. Ciccolini, M. Hamimed
Partners: Institutional: Inserm, AMU, APHM, IPC, CHU Nice.
Clinical need:
High dose Busulfan (HD-Bu) is the backbone of conditioning regimen in patients scheduled for bone-marrow transplant. HD-Bu leads to up to 30% of toxic death. To what extent PK-guided dosing a sequential regimen to reach a target exposure of AUC = 16000 uM.min-1 could improve the risk-benefit ratio of HD-Bu is yet to be elucidated.
Objectives:
To evaluate the safety and efficacy of sequential administration of high-dose Busulfan as part of conditioning regimen in leukemia patients scheduled for bone marrow transplant.
Data
PK data will be generated upon blood sampling in a longitidinal fashion (i.e., D-7, D-5, D-4). Clinical data (efficacy, toxicity per CTCAE grading) will be collected from start of the conditioning regimen up to 5-years after the transplant.
Methodology,
1. Buseq is a phase 2, multicentric study registered as 2020-003392-17.
2. Busulfan will be assayed for plasma concentrations by a fully validated LC-MS/MS method.
3. Individual PK parameters will be estimated using a Bayesian procedure and a home-made pop-PK matrix using a compartmental approach. Parameters identification (Kel, Vd, Cl, T1/2) and estimation of exposure metrics (AUC) will be performed on the Kinetic-Pro software.
4. PK/PD relationships will be investigated on Monolix software.
Funding
IPC.
MOIO:
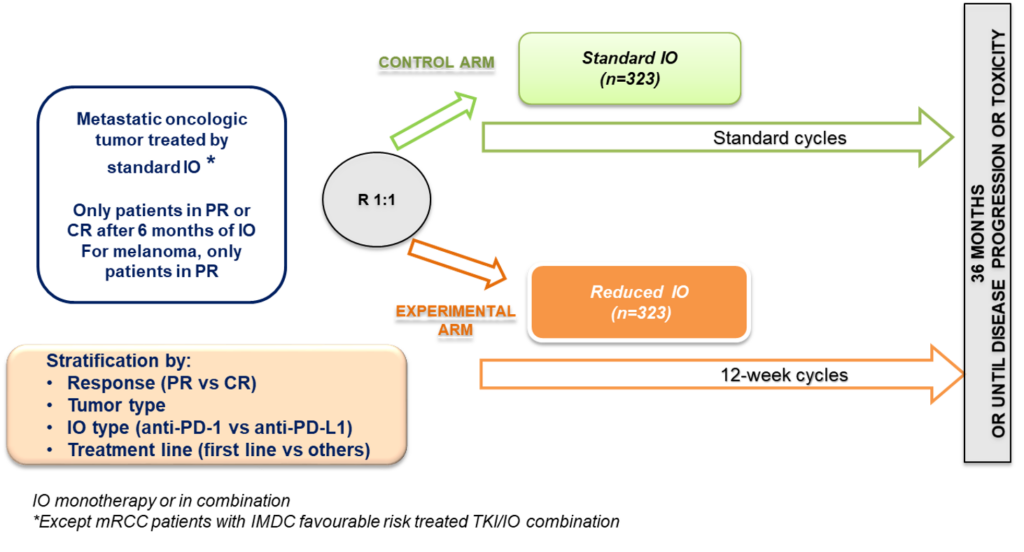
Randomized phase III trial of standard immunotherapy (IO) by checkpoint inhibitors, versus reduced dose intensity of IO in patients with metastatic cancer in response after 6 months of standard IO
P.I.: G. Gravis (IPC)
COMPO members involved: J. Ciccolini, M. Hamimed
Partners: IPC, Institut de cancérologie de l’Ouest, Centre François Baclesse, Centre Jean Perrin, CHU Henri Mondor, Centre Hospitalier Intercommunal, Centre Georges François Leclerc, hôpital Saint Louis, CAL, Institut Curie, Centre Eugène Marquis, Institut Claudius Regaud – IUCT-Oncopole, La Pitié Salpétrière, Hôpital Européen Georges Pompidou, Institut Ste Catherine, Hospices Civils de Lyon, CHU Besançon, CHU Timone, CHU Bretonneau, CHU Poitiers, ICANS, Hôpital Foch.
Clinical need:
Immune checkpoint inhibitors are currently overdosed with respect to the required thershold in plasma concentrations associated with target engagement.To what extent extended intervals between administration would ensure adequate exposure levels is yet to be determined
Objectives:
To demonstrate the non-inferiority in term of PFS of administration of reduced dose intensity of IO versus standard IO for patients in response after 6 months of standard IO. Endpoint: Progression-free survival calculated from the date of randomization to the date of first progression or death from any cause.
Data
PK data will be generated upon blood sampling in a longitidinal fashion at baseline, 3 months, 6 months and upon signs for progressive disease. Clinical data (efficacy, safety) will be collected from start of the randomization and up to 2-years.
Methodology,
1. MOIO is a phase 3, randomized, multicentric non-inferiority study registered as NCT05078047
2. Immune checkpoint inhibitors will be assayed for plasma concentrations by a fully validated LC-MS/MS method.
3. Individual PK parameters will be estimated using available pop-PK parameters using standard compartmental approach. Parameters identification (Kel, Vd, Cl, T1/2) and estimation of exposure metrics (AUC, trough levels, Cmax) will be performed on the Monolix software.
4. PK/PD relationships will be investigated on Monolix software.
Funding
INCa, BMS.
CYTA-PGx:
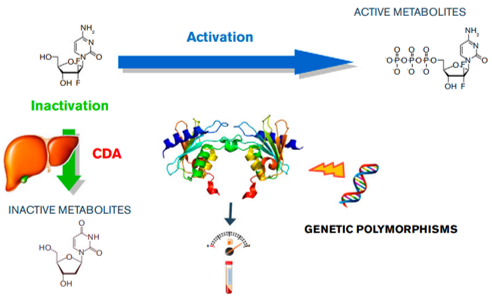
Pharmacokinetics & pharmacogenetics of cytarabine in adult AML patients: impact of the cytidine deaminase genetic polymorphism.
P.I.: R. Fanciullino (COMPO)
COMPO members involved: J. Ciccolini, M. Hamimed, M. Donnette
Partners: APHM Marseille, Centre Antoine Lacassagne Nice
Clinical need:
Cytarabine is a mainstay for treating AML patients as part of the canonical 7+3 protocol. Still, life-threatening toxicities and erratic efficacy are reported. To what extent unpredictable clinical outcome with cytarabine could come from inter-patient variability is yet to be found. To what extent changes in Cytidine Deaminase status, the liver enzyme responsible for the detoxification of cytarabine, could be the culprit for poor clinical outcome, is yet to be found as well.
Objectives:
To evaluate the impact of CDA phenotype (i.e., PM, EM, UM) and genotype and the dCK genotype on the PK parameters and exposure levels of cytarabine in adult patients treated for AML as part of 7+3 protocol.
Data
PK data will be generated upon 7 blood sampling during the induction phase (i.e., on the 7th day). Clinical data (efficacy, toxicity per CTCAE grading) will be collected
Methodology,
1. CYTA-PGx is a multicentric study registered as 2017-00070-53.
2. Cytarabine and its metabolite will be assayed for plasma concentrations by a fully validated LC-MS/MS method.
3. CDA status will be established using a double: phenotyping + genotyping (sequencing) approach. DCk status will be evaluated by sequencing.
4. Individual PK parameters will be estimated using a compartmental approach. Parameters identification (Kel, Vd, Cl, T1/2) and estimation of exposure metrics (AUC) will be performed on the Monolix software.
5. PK/PD relationships will be investigated on Monolix software and statistical analyse on R.
Funding
AORC Junior Grant (APHM).
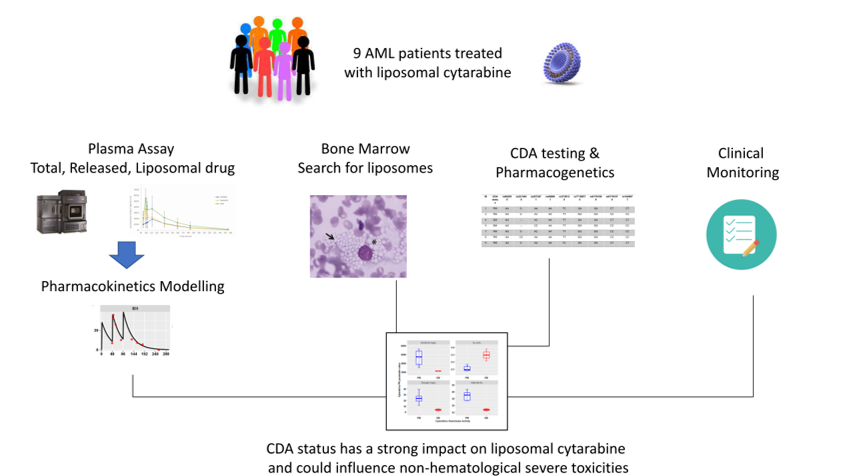
CYTA-PGx: Ancillary Study on CPX-351 (Vyxeos(R))
Pharmacokinetics & pharmacogenetics of liposomal cytarabine in adult AML patients: impact of the cytidine deaminase genetic polymorphism.
P.I.: R. Fanciullino (COMPO)
COMPO members involved: J. Ciccolini, M. Hamimed, M. Donnette
Partners: APHM Marseille
Clinical need:
CPX-351 (Vyxeos) is a liposomal formulation of cytarabine associated to daunorubicine. To what extent the PK profile of CPX-351 could be affected by CDA genetic polymorphism is unknown.
Objectives:
To evaluate the impact of CDA phenotype (i.e., PM, EM, UM) and genotype on the PK parameters and exposure levels of CPX-351 in adult patients treated for AML.
Data
PK data will be generated upon 7 blood sampling during the induction phase (i.e., on the 7th day). Clinical data (efficacy, toxicity per CTCAE grading) will be collected
Methodology,
1. Ancillary study of the protocol CYTA-PGx registred as 2017-00070-53.
2. Liposomal, released and total cytarabine will be assayed by LC-MS/MS technique.
3. CDA status will be established using a double: phenotyping + genotyping (sequencing) approach.
4. Individual PK parameters will be estimated using a compartmental approach. Parameters identification (Kel, Vd, Cl, T1/2) and estimation of exposure metrics (AUC) will be performed on the Monolix software.
5. PK/PD relationships will be investigated on Monolix software and statistical analyse on R.
Funding
AORC Junior Grant (APHM).
TORNADO: TOols for Rational NAnoDrugs Optimization: development of a physiologically-based pharmacokinetic model for nanomedicines
P.I.: F. Gattatteca (COMPO)
COMPO members involved: J. Ou (PhD student), M. Mahdjoub (M2 intern), A. Rodallec
Partners: CERIMED Marseille (P. Garrigue), MINT Angers (S. Legeay, E. Roger)
Clinical need: Pharmacokinetics of nanomedicines is poorly understood, which impairs their efficient development and clinical translation
Objectives: To develop a generic physiologically-based pharmacokinetic (PBPK) model to describe, understand and predict the PK and biodistribution of various types of nanomedicines
Methodology: Available in vivo data of PK and biodistribution of various types of nanomedicines will be integrated to develop a PBPK model dedicated to nanomedicines, encompassing the main mechanisms of PK of nanomedicines (endocytosis, clearance by mononuclear phagocyte system, enhanced permeability and retention). The corresponding parameters will be measured in vitro. In silico models will be developed to predict them based on the characteristics of the nanovector (type, composition, size, charge, shape).
Funding: AMU doctoral grant
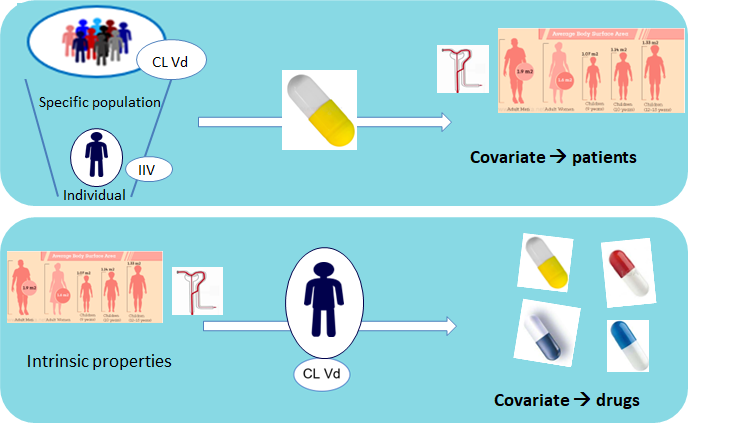
PKID: Population pharmacokinetic approach centered on the patient: a patient pharmacokinetic ID from one drug to predict the PK of the next one
P.I.: F. Gattacceca (COMPO)
COMPO members involved: A. Deschamps (PhD student)
Partners: APHM Marseille (R. Guilhaumou)
Clinical need
When starting a treatment with a new drug, PK data collected for another drug are of no use to predict the PK of the new drug.
Objectives
To use PK data from 2 drugs sequentially administered to the same patients (drug switches) to develop a combined population PK model allowing the prediction of the PK of the second drug from the parameters estimated for the first one.
Data
PK data from intensive care units patients experiencing drug switches have been collected.
Methodology
1. Proof of concept with drugs of the same class (beta-lactams)
2. Application to drugs of different classes (vancomycin and methotrexate)
Funding
APHM (Hospital pharmacy resident)