ANR DALLISH — Data Assimilation and Lattice LIght SHeet imaging for endocytosis and exocytosis pathway modeling in the whole
(PRC – Défi 7 – Axe 5 / Programme CES 23 2016)
CONSORTIUM
Inria
- EPC Serpico (Rennes) (coordinator: C. Kervrann) is an Inria team involved in the development of computational methods for biological imaging and analysis with a focus on protein trafficking analysis at the single cell level.<li>
- EPC Fluminance (Rennes) is an Inria team dedicated to the study of methods for the measurement and analysis of fluid flows from image sequences.
- EPC Beagle (Lyon) focuses on computational cell biology, at the interface between biology, biomathematics and computer science.
Institut Curie
- U1143 INSERM / UMR 3666 CNRS-Institut Curie, Chemical Biology of Membranes and Therapeutic Delivery unit. The objective of the unit is to use the enabling power of synthetic chemistry to create novel chemical structures and innovative tools for the study and manipulation of membrane biological systems such as endocytosis to establish a competitive basis for discovery at the forefront of cognitive science and biomedical research.
- UMR 144 CNRS-UPMC-Institut Curie, Space-time Imaging of Cellular Dynamics and Organelles and Endomembranes team is a research team which develops approaches in order to decipher the dynamic coordination and organization of molecular complexes at the single cell level.
INRA
- MaIAGE research unit (Jouy-en-Joas) brings together applied mathematicians, computer scientists and bio-informaticians to tackle data analysis and modeling issues from molecular to population and landscape scales. MaIAGE is developing original mathematical or computational tools that can be either generic or motivated by a dedicated biological question.
EXPECTED RESULTS, ORGINALITY, NOVELTY
New paradigms and computational strategies for processing analyzing temporal series 3D fluorescence microscopy images, and deciphering biomolecule trafficking and intracellular transport in live cell imaging.
- Challenge #1: Dramatically improve image processing for 3D fluorescence imaging (LLS-M, Bessel beam, 3D TIRF) and develop software that could be used for larger studies in biological imaging
- Challenge #2: Develop cutting-edge quantification methods to analyze dynamics and interaction of molecules.
- Challenge #3: Demonstrate the impact of combined LLS-M and computational frameworks in biological studies with a focus on endocytosis / exocytosis mechanisms.
EXECUTIVE SUMMARY
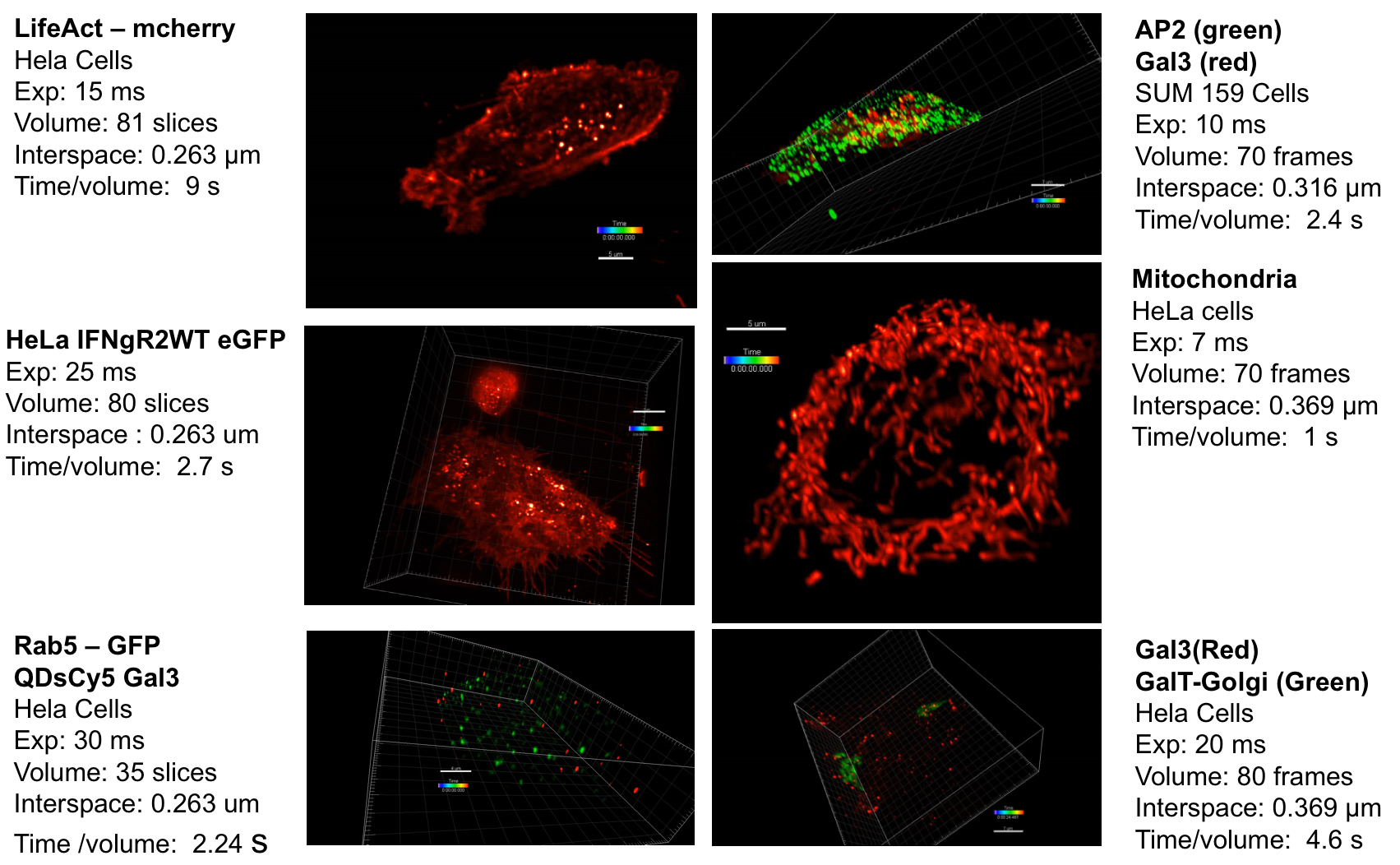
Context: Fluorescence imaging and microscopy has a prominent role in life science and medical research. It consists of detecting specific cellular and intracellular objects of interest at the diffraction limit (200 nm), using wide field as well as confocal microscopy, after tagging them with genetically engineered proteins that emit fluorescence. In this context, the past decade has witnessed a tremendous interest in the concept of Super-Resolution Microscopy (SR-M) and 3D fluorescence Light Sheet Microscopy (LS-M). One of the main reasons explaining this enthusiasm lies in the discovery of methods to break the diffraction limit established in optics several decades ago. Since, variants of SR-M (awarded by Nobel Prize in 2014) and LS-M have been investigated in an increasing number of biological studies. The so-called Lattice Light Sheet Microscopy (LLS-M) represents at present the novel generation of 3D fluorescence microscopes dedicated to single cell analysis, generating extraordinarily sharp, 3D images and videos. However, the usual conventional image processing algorithms developed for fluorescence microscopy are likely to fail to process the deluge of voxels generated by LLS-M instruments. The DALLISH project aims at improving the core of 3D image processing and quantification methods to face this computational challenge. The proposed methods will be used to decipher mechanisms involved in protein transport observed in LLS-M experiments.
Reconstruction of very huge 3D images: Inverse problems and image processing may be intractable with LLS-M because we are facing very large temporal series of volumes (200–1000 images per second for one 3D stack) acquired for several hours. These volume series represent several hundreds of Gigabytes if the LLS-M is used to study the 4D interplay of several proteins in the same cell at the same time. Accordingly, the usual methods need to be extended and novel strategies have to be found since computation is extremely heavy in LLS-M experiments. First, we will address the problem of image reconstruction (including deskewing) and deconvolution, which are the core and mainstream parts of LLS-M. All 3D data sets have to be deskewed to account for the 31.8° angle of the detection objective. Deconvolution of 3D images reduces blurring from out-of-focus light and enables quantitative analyses, but existing software for deconvolution is slow and expensive. We are aiming for parallelized methods that reconstruct and deconvolve 3D images faster than conventional software (few seconds per image) and run on a low-cost graphics processor board (GPU).
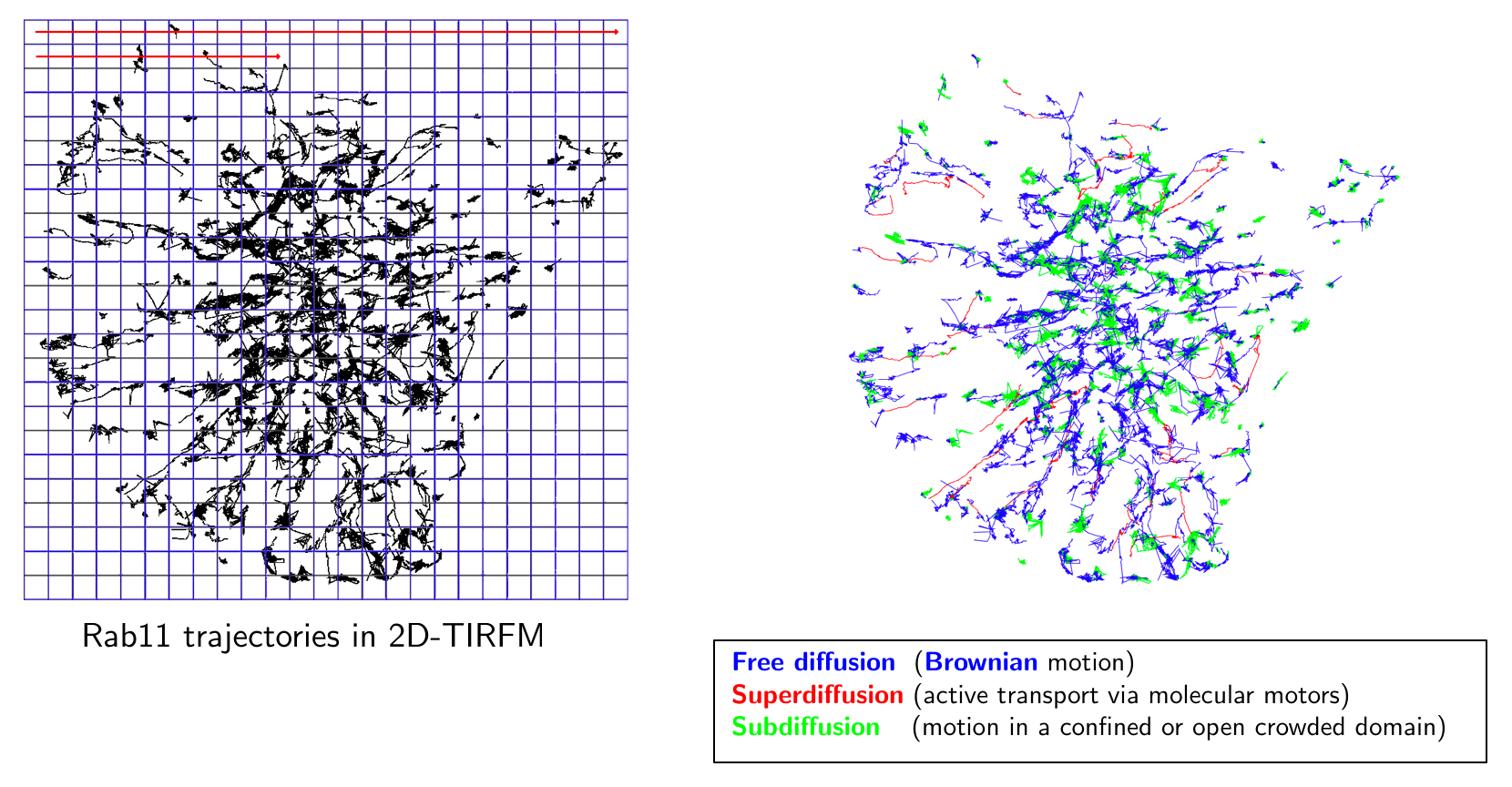
Image analysis of huge 4D images: Several image analysis methods need to be developed to quantify intracellular trafficking. First, we will study the problem of detection and segmentation of individual molecules/spots and extend the state-of-the art methods developed in 2D imaging to 3D LLS-M. Second, we will adapt the usual optical flow and tracking methods in fluorescence microscopy to meet the requirements in 4D LLS-M imaging. We will focus on the recent optical flow methods that combine local and global approaches and use the concept of estimator aggregation. An additional promising paradigm based on temporally varying object counting on multi-scale 3D spatial grids will be examined in the project. These segmentation and motion analysis methods and algorithms will be used to track endocytic and exocytic building blocks in time and 3D over the entire volume of a cell, such as to be able to follow individual transport vesicles and molecules propelled by motor activity, from the cell surface into the cell (endocytosis) or from endosomes to the cell surface (exocytosis).
Quantitative microscopy with biophysical models: Given image motion-based features and tracklets (small trajectories), we will investigate quantification methods to represent interactions between molecules and trafficking around three lines of research. First, we will study 3D space-time global and local object-based co-localization methods to quantify interactions between molecular species. Second, the dynamics of trajectories will be classified into three categories: Brownian motion, confined diffusion, directed diffusion. Therefore, statistical tests will be developed to replace conventional methods (e.g. MSD), which cannot reliably discriminate these three motion types. Moreover, the detection of regime changes occurring along trajectories will be modeled by generative models and analyzed in the Bayesian framework. Finally, given N tracks associated to N molecular species, stochastic models representing interactions between the molecular species will be also studied in the Bayesian statistical framework. Third, we will investigate approaches to estimate molecular mobility and active transport from the computed trajectories or optical flow descriptors. We will investigate the concept of super-resolution to provide spatially high-resolved maps of diffusion and active transport parameters based on stochastic models. An additional approach will be based on sparse image representation combined with biophysics to localize molecules at higher-resolution than the LLS-M resolution.
Endocytosis and exocytosis pathways analysis: Understanding the molecular and cellular mechanisms underlying membrane traffic pathways is central in fundamental cell biology and crucial to the treatment and cure of human disease: various human diseases caused by changes in cellular homeostasis arise through a single gene mutation; pathogenic agents such as viruses, bacteria, or parasites have evolved mechanisms to corrupt the host cell response to infection. Here, we propose to combine LLS-M, quantitative bioimaging, individual-based modeling and cell biology to assess the functional properties of molecular complexes and elucidate the role of key molecules involved in the endocytosis and exocytosis pathways. To this end, the algorithms, gathered in well-defined workflows, will be exploited to analyze experimental data composed of several series of proteins expressed at endogenous levels after genome editing. The framework will be quite flexible to adapt to a large range of biological studies and 4D microscopy modalities.
PUBLICATIONS
Publications of the members of the consortium
- A. Caranfil, C. Kervrann. Local temporal image correlation spectroscopy for sparse diffusion estimation in fluorescence imaging, Biophysical Journal, 2019. (submission)
- A. Salomon, C. Kervrann. Precise mapping of intracellular diffusion and drift from SPT data analysis, Biophysical Journal, 2019. (submission)
- S. Manandhar, P. Bouthemy, E. Welf, P. Roudot, C. Kervrann. 3D optical flow estimation combining 3D Census signature and Total Variation regularization, IEEE Int. Symp. Biomedical Imaging (ISBI 2020), 2019. (submission)
- V. Briane, M. Vimond, A. Salomon, C. Kervrann. A computational approach for detecting micro-domains and confinement domains in cells: a simulation study, Physical Biology, https://doi.org/10.1088/1478-3975/ab5e1d, 2019.
- S. Manandhar, P. Bouthemy, E. Welf, G. Danuser, P. Roudot, C. Kervrann. 3D flow field estimation and assessment for live cell fluorescence microscopy. Bioinformatics, btz780, https://doi.org /10.1093/bioinformatics/btz780, 2019.
- V. Briane, M. Vimond, C. Kervrann. An overview of diffusion models for intracellular dynamics analysis, bbz052, https://doi.org/10.1093/bib/bbz052, Briefings in Bioinformatics, 2019.
- V. Briane, C. C. Kervrann, M. Vimond. Statistical analysis of particle trajectories in living cells, Physical Review E, 97, 062121 (doi:10.1103/PhysRevE.97.062121), [http://hal.inria.fr/hal-01557705]
- S. Manandhar, P. Bouthemy, E. Welf, P. Roudot, C. Kervrann. A sparse-to-dense method for 3D optical flow estimation in 3D light-microscopy image sequences. http://www.rustburgpharmacy.com In Proc. IEEE Int. Symp. on Biomedical Imaging (ISBI’18), Washington, USA, April 2018.
- P. Roudot, L. Ding, K. Jaqaman, C. Kervrann, G. Danuser. Piecewise-stationary motion modeling and iterative smoothing to track heterogeneous motion in dense intracellular environments, IEEE Trans. Image Processing, 26(11):5395-5410, 2017. [http://hal.inria.fr/hal-01575754]
Joint publications of the consortium
- H.-N. Nguyen, C. Cauchois, V. Paveau, J. Salamero, L. Leconte, S. Prigent, C.A. Valdadez-Cruz, C. Kervrann. Sparse Hessian Variation for 3D and 3D+Time for fluorescence image restoration and background subtraction, Scientific Reports – Nature, 2019. (submission)
- Y. Lu, P. Hodara, C. Kervrann, A. Trubuil. An inverse problem approach to the probabilistic reconstruction of particle tracks on a censored and closed surface, Inverse Problems, 2019. (submission)
- S. Prigent, S. Dutertre, C. Kervrann. Empirical SURE-guided microscopy super-resolution image reconstruction from confocal multi-array detectors, IEEE Int. Conf. Acoustics, Speech, and Signal Processing (ICASSP 2020), 2019. (submission)
- V. Briane, M. Vimond, C. Valades Cruz, A. Salomon, C. Wunder, C. Kervrann. A sequential algorithm to detect diffusion switching along intracellular particle trajectories, Bioinformatics, btz489, https://doi.org/10.1093/bioinformatics/btz489btz489, 2019.
- F. Lavancier, T. Pécot, L. Zengzhen, C. Kervrann. Testing independence between two random sets for the analysis of colocalization in bio-imaging. Biometrics, https://doi.org/10.1111/biom.13115, 2019.
- A. Basset, P. Bouthemy, J. Boulanger, F. Waharte, J. Salamero, C. Kervrann. An extended model of vesicle fusion at the plasma membrane to estimate protein lateral diffusion from TIRF microscopy images, BMC Bioinformatics, 18:352, https://bmcbioinformatics.biomedcentral.com/articles/10.1186/s12859-017-1765-y, 2017.
- T. Pécot, L. Zengzhen, J. Boulanger, F. Waharte, J. Salamero, C. Kervrann. A quantitative approach for analyzing the spatio-temporal distribution of 3D intracellular events in fluorescence microscopy, eLife, 2018;7:e32311, https://elifesciences.org/articles/32311, doi:10.7554/eLife.32311),
2018. [http://hal.inria.fr/hal-01966817]
Participations to international conferences/workshops
25 (talks, posters)